Tips for K-REACH and TCCA Successful Compliance
original article from CIRS
The Act for the Registration and Evaluation of Chemicals (K-REACH) has come into force on 1 January 2015. K-REACH is originated and updated from the Toxic Chemical Control Act (TCCA). After the implementation of K-REACH, the section of new chemical substance regulation will be regulated under K-REACH. Hazardous substance control and chemical accident response will be controlled under TCCA.
Experts from CIRS have been studying TCCA since 2010, and have made a deeper study after the draft of K-REACH was published in 2012. Because K-REACH is originated from TCCA, we noticed that the registration of new substance of 1 – 10 ton per year in K-REACH is quite similar to that of TCCA, and TCCA seems even stricter than K-REACH. The requirements in TCCA for substances with amount of 1-10 ton per year have even reached the requirements of 10-100 ton per year in K-REACH, in terms of eye sensitivity, in vitro chromosomal aberration test and algae growth inhibition test. Therefore, if enterprises have succeeded in complying with TCCA, it will not be difficult in complying K-REACH.
As a professional regulatory service provider, CIRS have completed many TCCA registrations, which gives us experience in complying K-REACH. So far, CIRS has helped some companies complete several simplified registrations, and at the meantime, a number of standard registrations for substances of 10-100 ton per year are in progress.
CIRS will share our experience using FAQ to help enterprises gain a better understanding of K-REACH.
1. What department(s) is/are in charge of K-REACH?
Answer: K-REACH is managed by Chemical Management Association of South Korea (KCMA), National Institute of Environmental Research of South Korea (NIER) and Ministry of Environment of South Korea (MOE). MOE is the competent authority of K-REACH, KCMA is responsible for verification of registration exemption and NIER is responsible for the evaluation of registration.
2. What are the categories of K-REACH registration types and the differences between them?
Answer: K-REACH includes three registration types, simplified registration, standard registration and registration exemption
Simplified registration: Enterprises only need to provide basic information, and no extra tests are required. Registration fee includes administration fee and consulting fee. The duration is short; typically it can be completed within 15 business days. CIRS has already helped many companies complete the dossiers for simplified registration under K-REACH.
Registration Exemption: No requirement for extra tests either; enterprises only need to submit sufficient information to MOE. The registration duration is usually within 15 business days. Hints: If enterprises have already received the registration exemption under TCCA, they do not need to register for exemption under K-REACH again. However, if the exemption provision is changed, for example the Polymer of Low Concern (PLC) exemption has changed under K-REACH, under this situation, services from CIRS will definitely be a help to enterprises.
3. When does the K-REACH registration start?
Answer: Simplified registration, registration exemption and new substance standard registration started on 1 January 2015. You have to complete the registration before you can manufacture or import your product. Existing substance standard registration will start after the publication of the existing substance inventory, and enterprises shall complete the registration within 3 year. The first batch of existing substance inventory is expected to be finalized and published in June 2015. So far, 7 testing substances in “Pilot Project” have started to receive registration.
4. How does the K-REACH registration cost?
Answer: Same as EU REACH, K-REACH registration fee is consist of three parts: Data fee, administration fee and consulting fee. The data requirement for K-REACH is similar to that of REACH, if all the tests are carried out in GLP labs, the data fee for substance amount of 1-10 ton per year will be more than 350,000 RMB, and 10-100 ton per year will be more than 1,000,000 RMB. The data fee can be shared, and the more registrants share the fee, the less everyone needs to pay, which is similar to REACH. As to the administration fee, it will be much cheaper in K-REACH than that of in REACH. Registration exemption fee will be about 300 RMB, new substance simplified registration fee will be about 600 RMB and standard registration fee will be about 1,200 RMB.
5. What is the lab requirement under K-REACH?
Answer: For physicochemical property, except that the partition coefficient of n-ocanol/water has to be tested in GLP labs, the other data can all be gained in the normal labs. (Eco)toxicological data endpoints have to be done in the OECD GLP labs or the MOE nominated labs. The dossiers for K-REACH are required in Korean, so that enterprises are suggested to carry out the tests in the labs in Korea to avoid the extra translation fee. CIRS has visited a number of labs in Korea and has made collaboration partnerships with several enterprises. We can provide one-stop-shop K-REACH for our clients.
6. How long is the K-REACH standard registration operation duration?
Answer: The longest step in the K-REACH standard registration process is the GLP testing. Based on our previous experience, to complete all the tests (exclude the testing plan) for high tonnage level, the duration is between 6 to 9 months, dossier preparation is about 1 month, MOE review duration is about 1 month (exclude the situation of providing extra supplementary data), the total basic duration will be within 12 months. If enterprises have many existing data, the total basic duration is expected to be controlled within 6 months. If enterprises just comply with K-REACH by purchasing LOA, so the duration will be similar to REACH, that the time can be controlled within 3 months.
7. What are the K-REACH registration procedures?
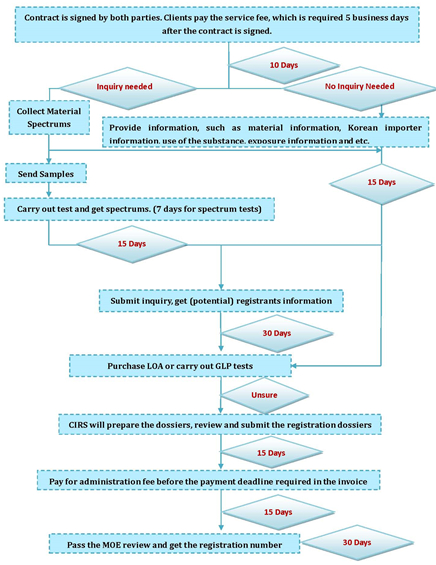
8. How enterprises should cooperate?
Answer: Similar to REACH registration, OR will carry out all the compliance work and enterprises only need to make decisions about how to comply, submit relevant information and pay relevant fees. CIRS can provide one-stop service from spectrum tests to dossier submission.
Contact Us
- CIRS China
Hangzhou CIRS Co. Ltd (CIRS China)
11F Building 1, Dongguan Hi-Tech Park, 288 Qiuyi Road, Binjiang District, Hangzhou 310052, China
Tel: +86-571 8720 6574 | Fax: +86-571 8720 6533
Email: service@cirs-reach.com

