
REACH Registration Deadlines
REACH requires all companies manufacturing or placing a substance on the EU market in quantities greater than 1t/year to register that substance with the European Chemicals Agency (ECHA). The deadline of REACH registration depends on the tonnage band of a substance and the classification of a substance.
REACH Registration Deadlines 2010, 2013 and 2018
Substances can be categorized into two groups under REACH: phase-in substances and non phase-in substances. Each group has different REACH registration deadlines.
Phase-in substances("existing substances") enjoy benefits of extended registration deadlines if pre-registered before Dec 2008. The principle is that the higher the tonnage, the earlier the registration deadline. Substances classified as CMR1/2 or R50/53(100t/y+)need to be registered before 30 Nov 2010 (see next diagram).
Non phase-in substances("new substances not covered by the definition of a phase in substance") need to be registered immediately before being placed in the EU market.
.
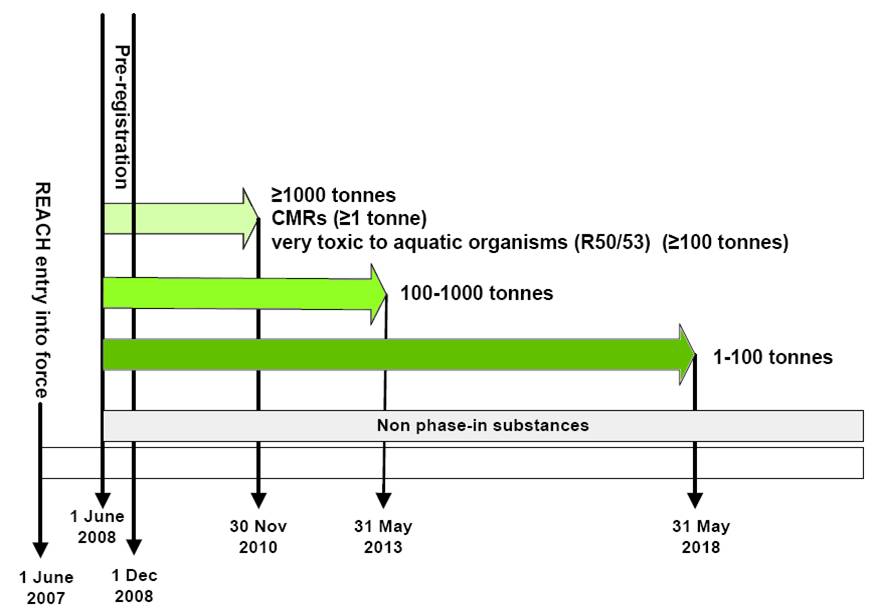
CMRs: carcinogenic, mutagenic or toxic to reproduction
Please click here to search whether your substances have been classified as CMR substances or R50/53 substances.
Definition of phase in substance
A substance which meets at least one of the following criteria:
- It is listed in the European Inventory of Existing Commercial Chemical Substances (EINECS).
- It was manufactured in the Community, or in the countries accepted to the European Union on 1 January 1995 or on 1 May 2004, but not placed on the market by the manufacturer or importer, at least once in the 15 years before the entry into force of this Regulation, provided the manufacturer or importer has documentary evidence of this.
- It was placed on the market in the Community, or in the countries accepted to the European Union on 1 January 1995 or on 1 May 2004, before entry into force of this Regulation by the manufacturer or importer and was considered as having been notified in accordance with the first indent of Article 8(1) of Directive 67/548/EEC but does not meet the definition of a polymer as set out in this Regulation, provided the manufacturer or importer has documentary evidence of this.
Phase-in substances that missed pre-registration cannot enjoy benefits of extended registration deadline and shall be registered immediately. However, first-time exporters/producers/importers who first began manufacturing or placing phase-in substances on the European market in excess of 1t/year after 1 Dec 2008, still can benefit from extended REACH registration deadlines by submitting a late pre-registration to the European Chemical Agency. Non-EU companies shall appoint REACH Only Representative to do so.
Quick Guidance To REACH Registration
- If you are a non-EU exporter or an importer in EU and have never done REACH pre-registration/registration before, you might need our REACH late pre-registration service;
- If you have appointed OR but wish to transfer OR to CIRS to help you complete registration at lower cost, please click here;
- If you intend to register a new substance or if you would like to register an existing substance but missed pre-registration, please click here - inquiry;
- If you have submitted your own pre-registrations and intend to register your substance as a member of joint submission, please click here - joint submission;
- If one substance is of strategic importance to you and no lead registrant or consortium is in place, please click here - lead registrant;
- If your substance is used as intermediate by your customers, please click here;
- If your substance is used for the purpose of product development and research(PPORD), please click here;
- If your substance is on SVHC candidate list, you might need our SVHC notification service.
Note 1: Intermediate registration and PPORD notification are much cheaper than REACH registration. If your substance meets the definitions of intermediate or PPORD, intermediate registration and PPORD notification are strongly recommended.
Note 2: If you place a chemical on EU market, you shall also comply with CLP regulation.
Note 3: companies shall start acting now and prepare for the coming reach registration deadlines in 2013.
About CIRS
CIRS a leading provider of comprehensive chemical compliance services for companies doing businesses in/with EU and China with a strong focus on chemical compliance.
With a strong presence in EU and China, CIRS has provided cost-effective regulatory support to over 3,000 companies while doing businesses in both the EU and China.
CIRS is the largest REACH only representative in the world. Since 2007, we have:
- pre-registered over 10,000 substances;
- acted as only representative for over 2,400 non-EU companies;
- served clients in more than 25 countries;
- registered over 145 substances to date;
- prepared over 1000 REACH SDS and CLP labels to date;
- submitted over 500 C&L Notifications to date;
CIRS is a recommended service provider by China Inspection and Quarantine Bureau, the US Mission to the EU and IDA. CIRS is also a member of Helsinki REACH Centre.
Why Choose Us?
Our full-range REACH registration services will provide end-to-end solutions to REACH registration. We have helped hundreds of non-EU firms and EU companies acquire over 180 REACH registration numbers up to date. The reasons to choose us include:
- Extensive substance specific registration experience;
- Broad communications with SIEF/Consortium;
- No hourly rates and hidden charges;
- Success guaranteed;
- Free regulatory updates and free consultations;
Please don't hesitate to contact us if you would like to find out whether you need to do reach registration or not and how much it might cost you. Our initial consultations are free.
Contact
- Europe Office
Unit 1 Ardee Business Park, Hale Street, Ardee, Co. Louth, Ireland
Tel : +353 41 9806 916 | Fax : +353 41 9806 999
Email: service@cirs-reach.com


_will_be_held_in_Shanghai.jpg)