
Medical Device and IVD Registration in China
Updated in January 2015
In China, Medical Devices (MDD) and In Vitro Diagnostic (IVD) reagents are regulated by following laws:
- The Regulations for the Supervision and Administration of Medical Devices (Decree No.650 2014)-main regulation
- Administrative Measures for Medical Device Registration(CFDA Order No. 4 2014)
- Administrative Measures for the Registration of In Vitro Diagnostic Reagents(CFDA Order No. 5 2014)
- The Provisions on Class I Medical Device Notification (CFDA Notice No.26 2014)
- The Provisions of Medical Device Classification (classification catalogue of medical devices)
- Administrative Rules for the Instructions and Labels of Medical Devices(CFDA Order No. 6 2014)
- The Guidelines for the Instructions of In Vitro Diagnostic Reagent Preparation (CFDA Notice No. 44 2014)
- Administrative Measures for the Supervision of Medical Device Manufacturing(CFDA Order No. 7 2014)
- Administrative Measures for the Supervision of distribution of Medical Devices(CFDA Order No. 8 2014)
- GMP-the Quality Management Practices for Medical Device Manufacturing (CFDA order No. 64 2014)
- GSP- the Quality Management Practices for Medical Device Distributing(CFDA order No. 58 2014)
- GCP- the Quality Management Practices for Medical Device Clinical Trial (SFDA notice No. 68 2012
Any Medical Device or In Vitro Diagnostic reagents manufacturer seeking to market their product in China must apply for and acquire the Medical Device Registration Certificate from China Food and Drug Administration (CFDA). Foreign companies should appoint local legal agent and service agent to deal with registration and after-sales service if they are not subsidiary or representation office in China.
How to determine the classification
Medical Devices (including IVD) are divided into three managing categories: class I, class II and class III medical devices based on the different risk level. There are higher controlled requirements if the product classified as higher level, such as class II or class III, and there are different requirements for each level as well. So the confirmation of the classification is the most important process and the first step to start the medical device registration.
The classification should be determined by the combined judgment from four aspects: intended use, structural characteristics, form of operation and intended for use. If you would like to get the classification and registration proposal for your product, please complete this order form and send it to Edwin.wen@cirs-group.com.
The Catalogue of medical dev ice classification code and name can be found here.
How to market your medical devices in China
The first important aspect of marketing your medical devices in China is to know how to process, CIRS medical device regulatory team will perform as your local regulatory staffs to assist with you to market your product in China step by step.
Registration scope and type for overseas medical devices
Any healthcare product meets the definition of medical device or IVD under CFDA regulations and is being to enter the Chinese market are required to register in China.
Definition of medical device
"Medical devices" are defined as any instrument, apparatus, appliance, material, in vitro diagnostic reagents and calibration substances and other similar substances and related articles, including the needed computer software. Its main effectiveness is achieved via physics ways and so on. It does not achieve its principal action in or on the human body by means of pharmacology, immunology or metabolism, but which may be assisted in its function by such means; the use of which is to achieve the following intended objectives:
- Diagnosis, prevention, monitoring, treatment or alleviation of disease;
- Diagnosis, monitoring, treatment, alleviation of or compensation for an injury or handicap conditions;
- Investigation, replacement or modification for anatomy or a physiological process;
- Life support or maintenance;
- Control of conception;
- Offer information for medical or diagnosis purpose via inspecting the human samples.
Registration type
Medical Devices |
Approval |
Testing |
Clinical Trial |
Authorities |
Deadline |
Class I |
Record |
Self-testing |
N/A |
CFDA |
Pre-market |
Class II |
Initial Registration |
Required |
Required |
CFDA |
Pre-market |
Updates |
TBA |
TBA |
CFDA |
Within 1months after being updated |
|
Renewal |
TBA |
N/A |
CFDA |
6 months before the date of certificate expired |
|
Class III |
Initial Registration |
Required |
Required |
CFDA |
Pre-market |
Updates |
TBA |
TBA |
CFDA |
Within 1months after being updated |
|
Renewal |
TBA |
N/A |
CFDA |
6 months before the date of certificate expired |
How to process the medical device registration in China
Data Requirements for Medical Device Registration
- Application form
- Documents for evidence
- The document to prove the safety and effectiveness of medical device
- Summary related to the registered product
- Study report (product designed, developed and verified report)
- Manufacturing process explanation
- Clinical evaluation documents
- Product risk assessment report
- Document of product technical requirements (former product standards)
- Product test report for registration
- Draft instructions and labels
- Self assurance statement
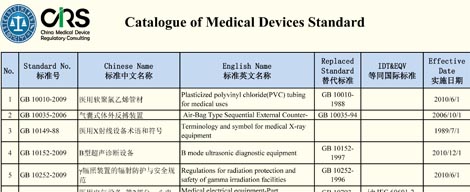
How to prepare product technical requirements (former product standards)
The product technical requirement for medical device shall be compiled according to the China related national standard of medical product. It is the most important document for product safety and effectiveness evaluating and the appendix of the medical device registration certificate.
The Catalogue of Medical Devices Standard can be found here, or contact Mr. Wen (Edwin.wen@cirs-group.com) to search the relevant product standard.
How to confirm the clinical trial requirement in China
Companies can check the clinical trial requirement step by step as follows:
GMP and GSP implementation timelines
Type |
Implementation date |
|
GSP |
All medical device distributing |
Dec, 12th 2014 |
GMP |
Sterile and implantable medical devices manufacturing |
Jan, 1st 2011 |
New establishment or update of manufacturing license for class III medical devices |
Oct. 1st 2014 |
|
Class III medical devices manufacturing |
Jan, 1st 2016 |
|
All medical devices manufacturing |
Jan, 1st 2018 |
|
Risk Assessment & Management
In medical device industry, the risk management is a vital part of all your company’s processes, it involves in the entire lifecycle of a device. There are different risks raised from each process of a device marketed in China: market risk, regulatory risk and product quality risk. To ensure your company gets a safe and effective product to entry into Chinese market on time and reduce the regulatory risk, a successes implementation of risk assessment and management would be a best support for your business in China.
How to get the free registration proposal
If you would like to get the free proposal for medical device registration in China, please complete the Application Form and send it to Edwin.wen@cirs-group.com, we will response to you upon your request.
Our Services
The following is a list of our services. Please contact us if you have any questions regarding your project.
- Pre-market Investigation & Analysis
- Medical Devices Registration & Approval
- Clinical Trial Consulting
- Manufacturing and Distributing License Approval
- Quality Assurance & Compliance
- Risk Assessment & Management
- Customs Clearance
- IPR Protection in China
Contact us
CIRS China
11F Dongguan Building, 288 Qiuyi Road, Binjiang District, Hangzhou, China, 310052
- Mr. Edwin Wen China office
Tel: +86 571 8720 6541 | Fax: +86 571 8720 6533
Email: Edwin.wen@cirs-group.com - Mr. Michael Petersen China office
Tel: +86-571 8720 6559 | Fax: +86-571 8720 6533
Email: Michael@cirs-group.com




