KKDIK was published on 23 Jun. 2017 by Ministry of Environment and Urbanization of Turkish Republic and formally came into effect on 23 Dec. 2017. The regulation shares many similarities with EU REACH, thus it is also called Turkey REACH.
Scope of Registration
- Substances in quantities of one ton or more per year manufactured in or imported into Turkey;
- Substances in mixtures in quantities of one ton or more per year manufactured in or imported into Turkey;
- Substances in articles manufactured in or imported into Turkey which are intended to be released under normal or reasonably foreseeable conditions of use and the quantity totals 1 ton per year or more.
Who shall Complete KKDIK Registration?
- Turkey manufacturers and importers of substances;
- Turkey manufacturers and importers of articles;
- Non-Turkey manufacturers of substances, mixtures and articles must perform the registration obligations under KKDIK through only representatives (OR) based in Turkey.
Timeline for Registration/Pre-registration
KKDIK Registration can be divided into two steps:
Step 1: Pre-registration’
Step 2: Registration.
Details are as follows:
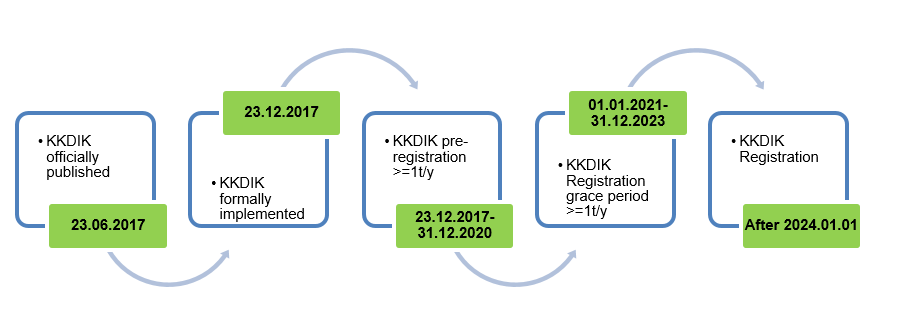
Notes:
- KKDIK was officially published on 23 Jul. 2017 and came into effect on 23 Dec. 2017. Preregistration obligations apply to substances manufactured or imported in quantities of one tonne or more per year per manufacturer or importer. The deadline of pre-registration is 31 Dec. 2020.
- The registration grace period is between 1 Jan. 2021 and 31 Dec. 2023. Enterprises who have pre-registered their substances before 31 Dec. 2020 can use the pre-registration number for manufacturing or importing during the period. However, Enterprises that have not completed pre-registration before 31 Dec. 2020 must complete registration, or they are not allowed to manufacture or import during the grace period;
- From 1 Jan. 2024, substances must finish registration before they’re manufactured or imported;
Information Required for Pre-registration
Substance information (such as substance name, CAS No., EC No., substance structure, etc)
Registration Type
Registration type | Tonnage band | Data requirements |
On-site isolated intermediates under strictly controlled conditions | >=1t/y | Existing data only |
Transported isolated intermediates under strictly controlled conditions | >=1t/y | Existing data only |
>=1000t/y | KKDIK Annex VII | |
Registration of regular substances
| >=1T/Y | A) 1-10t/y standard registration, all data specified in Annex VII; B) 1-10t/y, The information on physicochemical properties specified in Annex VII, for substances which do not meet either of the criteria specified in Annex 3; |
>=10T/Y | KKDIK Annex VII+VIII | |
>=100T/Y | KKDIK Annex VII+VIII+IX | |
>=1000T/Y | KKDIK Annex VII+VIII+IX+X | |
Note: for >=10t/y regular registration, a chemical safety report (CSR) is needed as well. | ||
Note:
1. Intermediates can be divided into 3 categories: Non-isolated intermediates, on-site isolated intermediates, transported isolated intermediates.
Non-isolated intermediates: an intermediate that during synthesis is not intentionally removed (except for sampling) from the equipment in which the synthesis takes place.
On-site isolated intermediate: an intermediate not meeting the criteria of a non-isolated intermediate and where the manufacture of the intermediate and the synthesis of (an)other substance(s) from that intermediate take place on the same site, operated by one or more legal entities;
Strictly controlled condition: Similar to EU REACH; refer to the requirements under EU REACH;
2. Terms of ANNEX III:
a) substances for which it is predicted (i.e. by the application of (Q)SARs or other evidence) that they are likely to meet the criteria for category 1A or 1B classification in the hazard classes carcinogenicity, germ cell mutagenicity or reproductive toxicity or the criteria in Annex XIII;
(b) substances:
(i) with dispersive or diffuse use(s) particularly where such substances are used in consumer 3 mixtures or incorporated into consumer articles; and,
(ii) for which it is predicted (i.e. by application of (Q)SARs or other evidence) that they are likely to meet the classification criteria for any health or environmental hazard classes or differentiations under By-law on Classification, Labelling and Packaging of Substances and Mixtures.
Polymers: Similar to EU REACH, polymers is exempted from registration under KKDIK. However, monomers or other reactant of polymers shall also get registered when they meet certain conditions.
Definition of polymer: a substance consisting of molecules characterised by the sequence of one or more types of monomer units, distributed over a range of molecular weights wherein differences in the molecular weight are primarily attributable to differences in the number of monomer units and comprises the following:
1) A simple weight majority of molecules containing at least three monomer units which are covalently bound to at least one other monomer unit or other reactant; and
2) Less than a simple weight majority of molecules of the same molecular weight.
Monomer: a substance which is capable of forming covalent bonds with a sequence of additional like or unlike molecules under the conditions of the relevant polymer-forming reaction used for the particular process;
Polymers can exempt from registration. But if their monomers and other reactants fulfill the following conditions, registration is required:
a) The polymer consists of 2 % weight by weight (w/w) or more of such monomer substance(s) or other substance(s) in the form of monomeric units and chemically bound substance(s);
b) The total quantity of such monomer substance(s) or other substance(s) makes up one ton or more per year.
KKDIK and EU REACH Regulation
Compared with EU REACH, the data review under KKDIK is much stricter than that under EU REACH. Under KKDIK, the registration dossiers must be prepared and uploaded to KKS system by the “Chemical Assessment Expert”. But, to submit KKDIK Pre-registration of the substances, the “Chemical Assessment Expert” is not needed.
Our Services
- KKDIK Regulatory Compliance Consulting and Training;
- KKDIK OR Service;
- KKDIK Pre-registration;
- KKDIK Registration;
- Turkey CLP Regulation Regulatory Compliance Consulting and Training;
- Turkey CLP Regulation OR Service;
- C&L Notification under Turkey CLP Regulation;
- Turkish SDS and Labeling Preparation;

