In order to promote the registration of foods for special medical purposes, former CFDA has revised the Stability Research Requirements for Foods for Special Medical Purposes (trial)( 2017 revision), which was released and implemented on September 5, 2017. The purposes of stability research are to obtain the change rules of product quality characteristics over time under certain environmental factors. The research can also provide supporting information for product formulation design, product processing, preparation and use, packaging specifications and packaging material selection, product storage conditions and shelf life determination.
CIRS group has analyzed and summarized the key requirements of the experiments as well as the registration materials mentioned in Stability Research Requirements for Foods for Special Medical Purposes (trial)( 2017 revision), which are demonstrated as following:
1. Stability experiments for FSMP
These stability experiments should be reasonably established and conducted depending on research purposes and product characteristics. Among them, the influencing factor test, accelerating test and long-term test are mandatory to conduct. While others could be selected to conduct, such as stability test while using, according to the specific characteristics of the product.
1.1 Accelerating test
Applicant shall take 3 batches of samples to conduct the accelerating test.
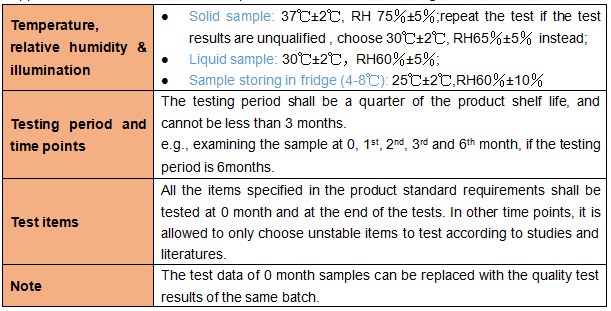
1.2 Long-term test
Applicant shall take 3 batches of samples to conduct the long-term test.
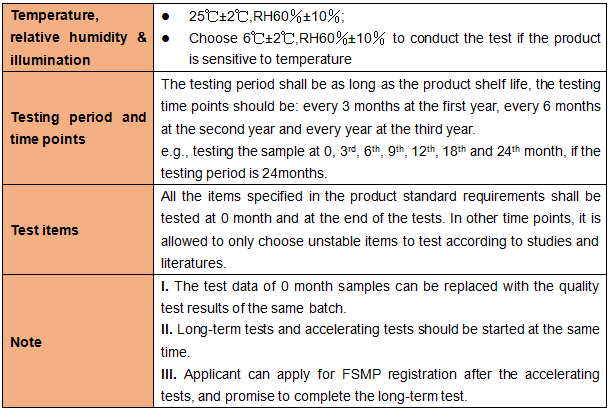
1.3 Influencing factor test
Applicant shall take 1 batch of samples to conduct the influencing factor test.
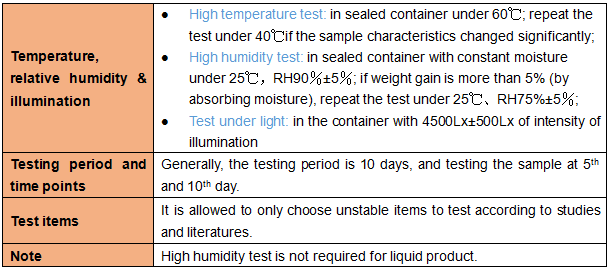
1.4 Other tests (stability tests while using)
The applicant can conduct additional tests selectively, including stability tests while using after opening the package, simulated tube feeding tests, product transportation tests and nipple tests (baby products), etc.
2. Requirements for registration materials
Applicant shall submit the materials related to stability research as following:
1) Name, specification, batch and batch quantity of the test samples, production date as well as start time of the tests;
2) Test conditions, such as temperatures, illuminations and relative humidity;
3) The name and quality requirements of packaging materials;
4) Test projects, analysis methods and limits of stability research;
5) All the analysis data obtained from the research (submit as the form of table);
6) The test results shall be expressed in specific values, in which the percentage of the nutrition component test results shall be marked with the first test results. The measurement unit shall conform to the relevant regulation, cannot use descriptions like ‘meet the requirements’. All test results and their relative standard deviation (RSD) shall be provided if multiple tests are carried out at a certain inspection point.
7) The major risks of the products during the storage period and the causes of the risks and their manifestations; selection basis of the stability experiments; the relationship between stability test results and selection of product storage conditions, shelf life, packaging materials and the product consumption methods. Analyzing the test results and obtaining the conclusion.
Note: The test reports and the study materials of stability research shall be submitted together with the product registration application (For infant formula for special medical purposes and nutritionally complete foods, the applicant can keep the records for further examination).
More information related to FSMP registration, please feel free to click the following link:
If you have any needs or questions, please feel free to contact us at service@cirs-group.com

