On November 12nd, 2019, SAMR issued the Guidelines for Naming of Health Food (2019 Version) (hereinafter named “Guidelines”), the Guidelines is applicable to the naming of the registration and filing products’ Chinese name. In order to help enterprises obtain the key information, CIRS interpreted the guidelines from two aspects: the prohibited contents in the Chinese product name, and the requirements for declaration and appraisal of Chinese product name.
1. Composition of product name
The product name of health food shall be composed of trademark name, general name and attribute name in proper order.

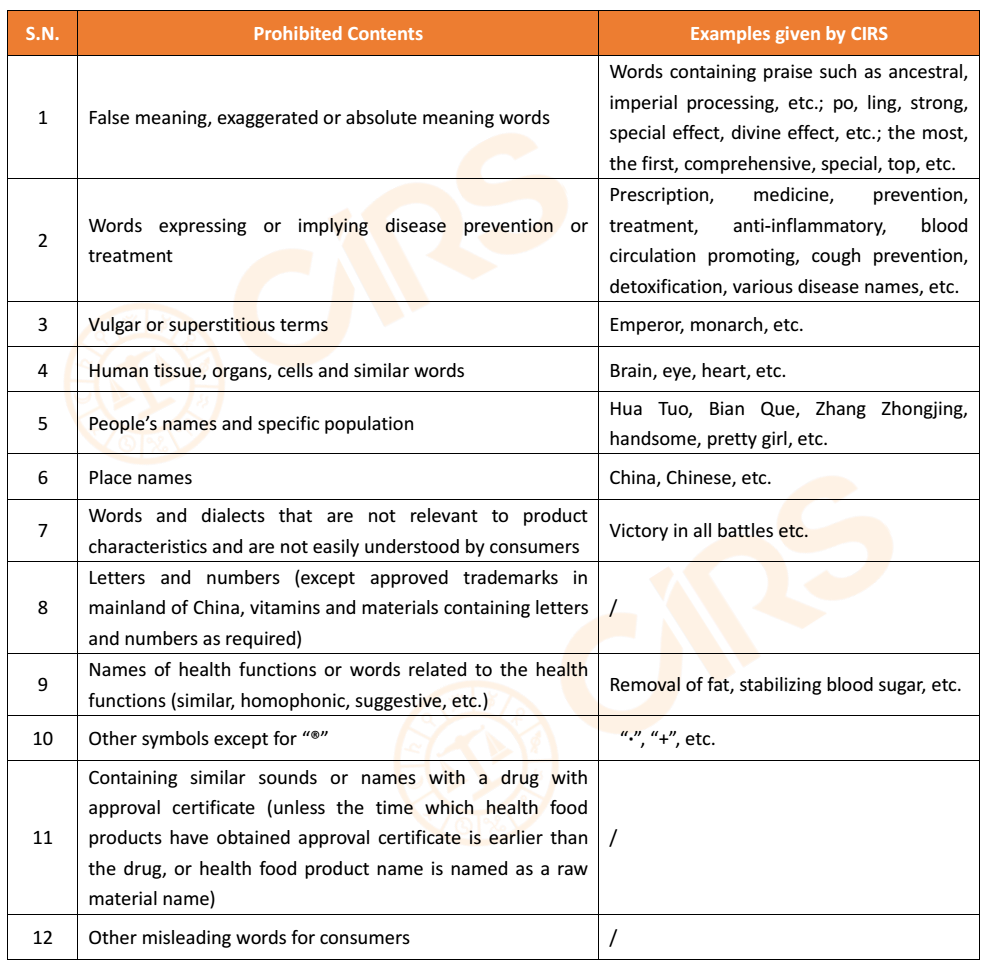
2. Prohibited contents in trademark and general name of product
3. Requirements for declaration and appraisal of product name
(1) Trademark name
The registered or non-registered trademarks in mainland of China can be used as trademark name. If a registered trademark is used in product name, the product category shall be consistent with the application scope of trademark listed in the trademark certificate. According to the experience of CIRS, the application scope of registered trademark to be used in health food product name, should belong to 5th or 30th Class under the trademark international classification.
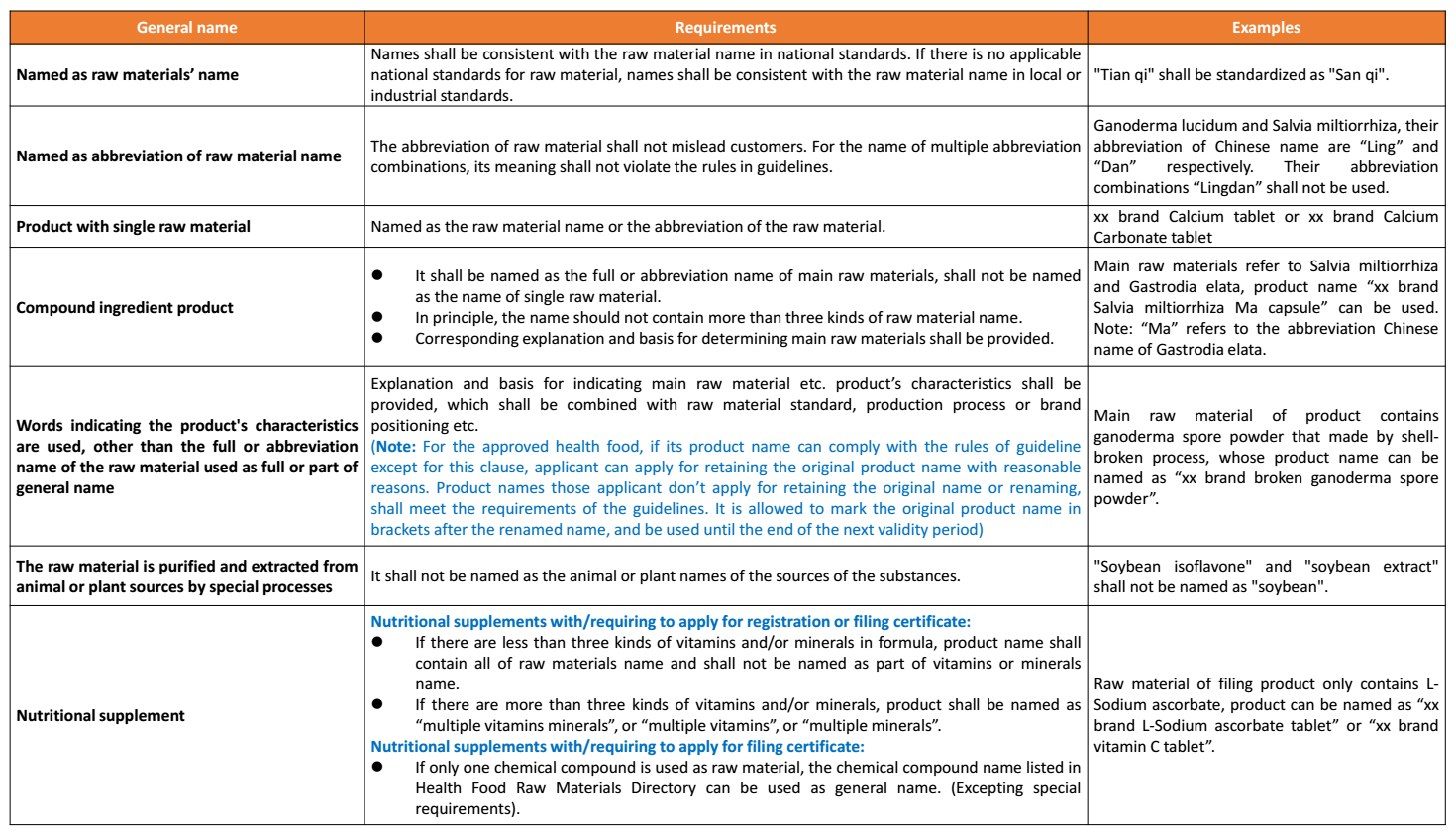
(2) General name
(3) Attribute name
- If there are applicable national, local or industrial standards for attribute name, attribute name is required to use the name listed in these standards, eg., beverage.
- If there are no applicable standards for product dosage form, attribute name shall be named according to the name specified in the “General Principles of Preparations” of Chinese Pharmacopoeia.
(4) Special mark on Chinese product name
- The special mark in the product name shall be marked with brackets after the attribute name, eg., if food flavor is used in formula, the specific flavor can be marked in product name; if there are kinds of food flavor in formula, only the main food flavor is allowed to be marked, eg., xx brand xx tablet (apple flavor);
- For nutritional supplement, specific population used in product name shall be consistent with product’s suitable crowds, eg., xx brand xx tablet (adults).
CIRS Comments
- For the health foods without approval certificates, the company shall name the product in strict accordance with the guidance;
- For the health foods have obtained the approval certificates, if the product name cannot comply with the guidelines, it is suggested to prepare the alteration application of product name in advance.
Original link: http://gkml.samr.gov.cn/nsjg/tssps/201911/t20191112_308443.html

