
Guidance on regulatory compliance approach for prepackaged food
updated in July 2014
According to the information published by the General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China (AQSIQ), CIRS's food regulatory team have found that there were high occurrences that batches of prepackaged foods were returned or destroyed by China Customs. Incompliant food additives, food raw materials, disqualified quality index and inappropriate Chinese labels were the main reasons for the returning or destroying of imported prepackaged foods in recent years. As a consequence much effort, time and financial resources of companies were unnecessarily wasted.
As a result CIRS has prepared this guidance for regulatory compliance approaches of prepackaged food to help food companies to export prepackaged food products to China successfully.
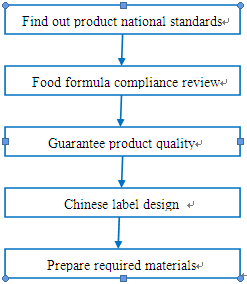
The procedure of ensuring regulation compliance for prepackaged food is demonstrated in the following chart:
Why and how to make your products compliant?
Step 1. Evaluate the national standards (GB) for prepackaged food.
If there is no applicable standard for your products, you should apply to the National Health and Family Planning Commission of the People’s Republic of China (NHFPC) for import permit of food without national standards. This process can be really difficult and as such CIRS could help you to find relevant national standards in accordance with product formula, production process and product feature.
Step 2. Product formula compliance review
You shall confirm that each ingredient of your prepackaged product including food raw material, food additive and nutrition enhancer is legitimate. For example additives are allowed to be used however the dosage much remains within limits according to national regulations. CIRS will help to decide whether your products ingredients can be legitimately used according to GB 2760, GB 14880 and national standards regarding product ingredients.
Step 3. Guarantee product quality
The criteria for pathogenic bacteria, contaminants and other quality index in food between domestic and overseas markets can be different. It is necessary to conduct pre-testing at a qualified laboratory in China to insure the product quality and to avoid possible rejection by China Customs.
Step 4. Chinese label design
Rectification would be proposed by Chinese Exit-Entry Inspection and Quarantine Bureau (CIQ) for substandard labeling. What is more, the products can be rejected by China Customs provided that the label is incompliant.
The label review requirements by CIQ are becoming stricter and companies may get adverse records in CIQ system because of the incompliant label from 1 July 2014. This can reduce the credibility or impact the reputation of a company and therefore can affect future product importation from this company later on.
CIRS will help you to review/make the Chinese label and check the icon and wording on the original packaging according to GB 7718, GB 13432 and GB 28050 to ensure compliance.
Step 5. Prepare required documents.
You need to prepare many documents for submission to CIQ such as, hygiene license, certificate of Origin, label sample both in Chinese and English,“4 plus 1” nutrition testing report and so on.
The five steps mentioned above could help you to reduce the risk of inspection by CIQ and State Administration for Industry and Commerce of the People's Republic of China (SAIC). If you would like to get more information about details and relevant regulations for free, please send us an email.
Contact Us
Ms. Cathy Yu, Senior Consultant, Food Safety Regulation
CIRS China 11F Dongguan Building, 288 Qiuyi Road, Binjiang District, Hangzhou, China, 310020
Tel : +86 571 8720 6564 | Fax : +86 571 8720 6533
Email: cathy.yu@cirs-group.com >
We welcome you to visit our new website in food area
http://www.cirs-group.com/food/

