Acne is most common among teenagers, with a reported prevalence of 70 to 87 percent. It is a skin condition that occurs when your hair follicles become plugged with oil and dead skin cells. Acne usually appears on your face, neck, chest, back and shoulders. Depending on its severity, acne can cause emotional distress and scar the skin. The earlier you start treatment, the lower your risk of lasting physical and emotional damage.
According to the CFDA keyword-based search result, 488 acne cosmetics, with word ‘acne’ in its name, are approved in recent 7 years (2009-Present). As is shown in Figure 1, the great demand of anti-acne cosmetics on the market is growing significantly in recent 4 years.
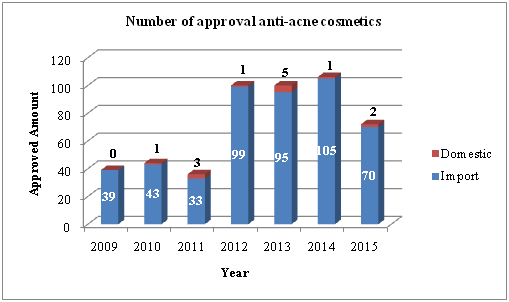
Figure 1 Number of approval anti-acne cosmetics
However, only import cosmetics and domestic special cosmetics are available on the CFDA website. Thousands of domesticnon-special cosmetics, without word ‘acne’ in its name but stating its acne treatment effect, shall be counted. In all, the anti-acne cosmetics will be a great potential market in China.
CIRS China has the full capacity to help foreign or domestic cosmetics companies get the
pre-market approval of anti-acne cosmetics. Detailed dossier requirements for the registration
ofanti-acnecosmetics in China are summarized as Table 1.
Table 1 Dossier requirements for anti-acne cosmetics registration in China
CIRS Comments:
1. Antibiotics and metronidazole have to be tested foranti-acne cosmetics. The testing results should be indicated in product safety and quality control file and product technical requirements.
2. The package of anti-acne cosmetics (including label and instructions) shall not have the claims expressing or implying the medical treatment, such as anti-inflammation, prescription and sterilization, etc.
3. ‘Professional’is not available to the advertisement of anti-acne cosmetics. According to the cosmetic nomenclatureguidelines, professionalcan be used for hair dye, hair perm and nails cosmetics only.
4. The authenticity and consistency of the documents provided should be guaranteed. For example, the related information(product name, name and address of manufacturer, test method, etc.) shown on the product safety and quality control file, testing reports, product technical requirement, production Process, package translation and Chinese labeling should be consistent .
5. The forbidden ingredients like glucocorticoid hormones (clobetasol propionate, hydrocortisone, betamethasone dipropionate, etc) will be the issues for this kind of cosmetics. It is suggested that the enterprise shall carry out the test of glucocorticoid hormonesso as to avoid the risk and control the quality.
6. The registration requirements for cosmetics with the claiming of acarus killingshall bethe same as anti-acne cosmetics.
Source:
http://www.sfda.gov.cn/WS01/CL0060/46154.html
http://www.sfda.gov.cn/WS01/CL0846/54432.html

