Definition of Cosmetics and Quasi-drugs in Japan
The Pharmaceutical Affairs Act defines cosmetics as "Articles with mild action on the human body, which are intended to be applied to the human body through rubbing, sprinkling or other methods, aiming to clean, beautify and increase the attractiveness, alter the appearance or to keep the skin or hair in good condition."
The Pharmaceutical Affairs Act defines “Quasi-drug” (医薬部外品) as an item for the purpose of:
(1) Preventing nausea and other discomfort.
(2) Preventing heat rash, soreness, etc.
(3) Encouraging hair growth or removing hair
(4) Exterminating and preventing mice, flies, mosquitoes, fleas, etc.
Category of Beauty Products in Japan
Beauty products are divided into cosmetics and quasi-drugs. For cosmetics, there are mainly 6 subcategories as below:
Subcategory | Example |
Perfume and eau de cologne | fragrance |
Makeup cosmetics | foundation creams, lipsticks and eye makeup |
Skin care cosmetics | facial cream, skin lotion, skin milk and cleansing cream |
Hair care products | hair dye, shampoo and hair treatment |
Special-purpose cosmetics | Sunscreen and shaving cream |
Soaps | - |
With regard to quasi-drugs, they includes deodorants, depilatories, hair growth treatments, hair dyes, perm and straightening products, as well as medicated cosmetics, such as whitening products, anti-aging products and oily skin or acne treatment products.
Who will be the responsible person of cosmetics quality and safety in Japan?
The importers (primary distributors) undertake the product quality and safety of cosmetics. Furthermore, the importers have to be the local Japanese company. A subsidiary of foreign company in Japan can be acted as a(n) importer or distributor as well. The importers need to appoint one or three responsible person(s) under the compliance of Good Quality Practice (GQP) and Good Vigilance Practice (GVP) to be in charge of the supervision of marketing, quality and safety control. Main responsibilities of importers (primary distributors) are as follows:
- Evaluating the results of production management and the quality control of cosmetics to be actually distributed
- Deciding whether to ship to the market by lot
- Having the records regarding whether to and where to ship the cosmetics and Keeping these records for five years.
- Establishing a system capable of providing accurate information in response to consumer inquiries, along with a system for handling complaints about product quality and the like as well as product recalls.
- Reporting the safety issue to the Minister of Health, Labour and Welfare within 30 days.
How to export cosmetics to Japan?
The importation of cosmetics is subject to the provisions of the Pharmaceutical Affairs Act When importing and distributing cosmetics, under the provisions of the revised Pharmaceutical Affairs Act, which went into effect as of June 1, 2009, the importer must obtain a primary distributor's license for cosmetics. The revised Act abolished the importer license classification. Any primary distributor that engages in the final packaging, labeling in the Japanese language, or storage of the imported product, is required to obtain a cosmetic manufacturer's license.
1) Flow chart of approval procedure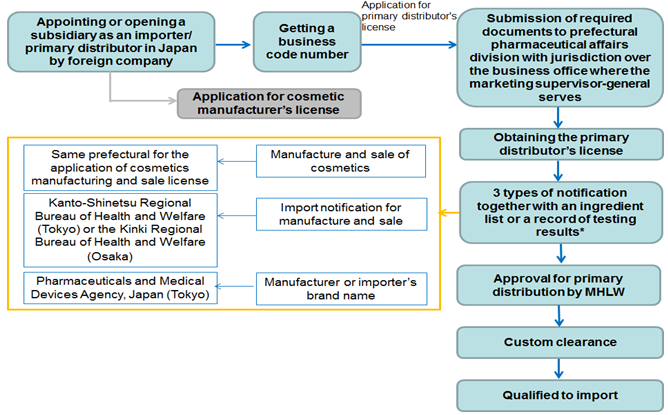
* If the provided ingredients comply with the Cosmetics Standards and all the ingredients are indicated on the labeling, approval for primary distribution by product item is not required. Nevertheless, products containing amounts of ingredients in excess of the limit level, or new ingredients without a history of prior usage, or which contain non-disclosed ingredients, must obtain primary distribution approval for each product item.
2) Required documents for the application of primary distributor’s license and cosmetics manufacturer’s license
- a. primary distributor’s license
- a copy of the corporate registration (in the case of a corporation);
- a list of duty specifications;
- a medical certificate specifying the applicant;
- documents certifying the qualifications of the marketing supervisor-general;
- an employment contract of the marketing supervisor-general;
- documents disclosing the quality management system;
- documents disclosing the post-marketing safety management system;
- a floor plan of the business office and storage facility.
b. cosmetics manufacturer’s license
- an outline of the physical facility;
- a floor plan of the manufacturing facility;
- documents certifying the qualifications of the responsible engineer;
- the employment contract of the responsible engineer;
- a copy of the contract with a testing laboratory (when used)
How to export quasi-drug to Japan?
Quasi-drugs are a unique product classification in Japan. A quasi-drug defined in Japan has minimal to moderate pharmacologic activity but is restricted in use to specific indications. A foreign manufacturer must appoint a drug marketing authorization holder (MAH) in Japan who performs all procedures with the MHLW on behalf of the applicant for the application of the marketing approval. Moreover, the MAH must have a quality representative, medical safety officer and General Manager who are qualified to take responsibility for release of the medicinal product in Japan.
Flow chart of approval procedure
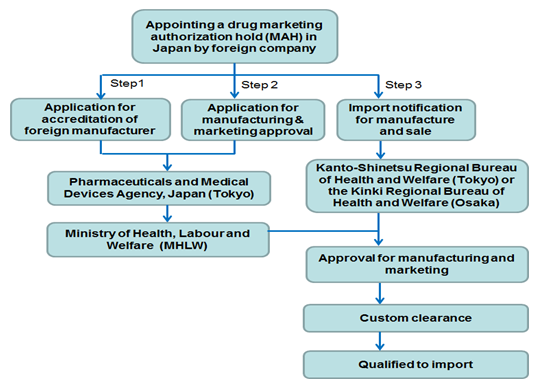
Note: 1) onsite-inspection of foreign manufacturing site required for GMP compliance review so as to get the accreditation of foreign manufacturer; 2) requirements for the application of manufacturing & marketing approval include the quality, efficacy and safety of each product, license of marketing authorization holder and accreditation certification of foreign manufacturer and GMP compliance.
Labelling requirements of cosmetics
According to Pharmaceutical Affairs Act, the container, packaging, or package inserts of cosmetics be labeled with the specified items so as to ensure appropriate usage, handling, quality, and to clarify liability. All must be expressed in the Japanese language. Labeling with false or potentially misleading expressions, and unapproved claims of effect -efficacy in labeling are prohibited. The items that should be indicated for cosmetics are as follows:
Cosmetics
[1] Product name by type
[2] Brand name
[3] Name and address of primary distributor
[4] Content (weight or capacity)
[5] Country of origin
[6] Manufacturing number or code
[7] List of ingredients as required by the Minister of Health, Labour and Welfare
[8] Expiration date, for a cosmetic designated by the Minister of Health, Labour and Welfare
[9] Precautions on usage or storage, for a cosmetic stipulated under the Enforcement Regulation
[10] Information contact
Note: for approval quasi-drugs, the MHLW allows the product to display its effectiveness for a result that has yet to be recognized. This allows companies to indicate that the product is "Medicated."
If you have any needs or questions, please contact us at service@cirs-group.com.

