1. Background
In accordance to Food Safety Law of the people’s Republic of China, companies who plan to use new substances (including new food contact resins and new food contact additives) in food contact materials or products, or to enlarge usage scope of existing food contact resins or additives on Chinese market shall submit the related safety assessment material to National Health Commission of the People's Republic of China (NHC, former NHFPC).
To meet the demand of industry, to ensure the safety of new food contact material, and to standardize new food contact material application procedure, former NHFPC has drawn up the ‘Administrative Measures on New Food Related Product’ which specifically stipulated the concept, application, acceptance, administration permission of new food contact substance.
2. Introduction of New Food Contact Substance
New food contact substances refer to new food contact resins and new food contact additives.
New food contact resin refers to substance intended to be used in food contact materials or products, however, it is not in the list of food contact national standards including GB4806.6, GB4806.10, and GB4806.11, nor is it listed in subsequent announcements issued by NHC (former NHFPC).
New food contact additive refers to substance intended to be used in food contact materials or products, however, it is not in the list of “GB 9685-2016 National Standard for the Uses of Food Additives in Food Contact Materials and Articles”, nor is it listed in subsequent announcements issued by NHC (former NHFPC).
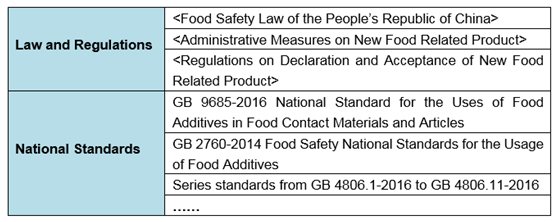
3. Relevant Laws and Regulation
4. Notification dossier
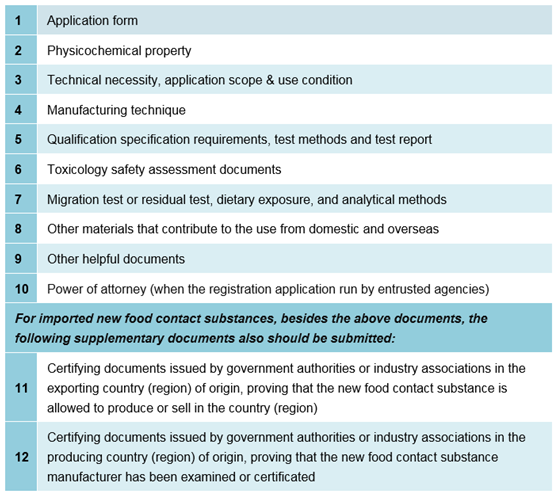
4.1 Required documents
4.2 Required toxicology test item
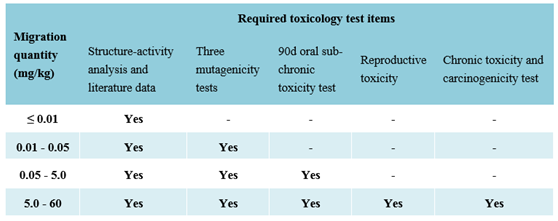
a. The three mutagenicity tests include: Ames test, bone marrow cell micronucleus test and vitro mammalian cell chromosome aberration test/vitro mammalian cell gene mutation test.
b. For the high-molecular polymer (average molecular weight above 1000 Dalton) shall provide the toxicological safety assessment documents of each monomer. However, toxicology test of polymer is not required.
5. Service procedure
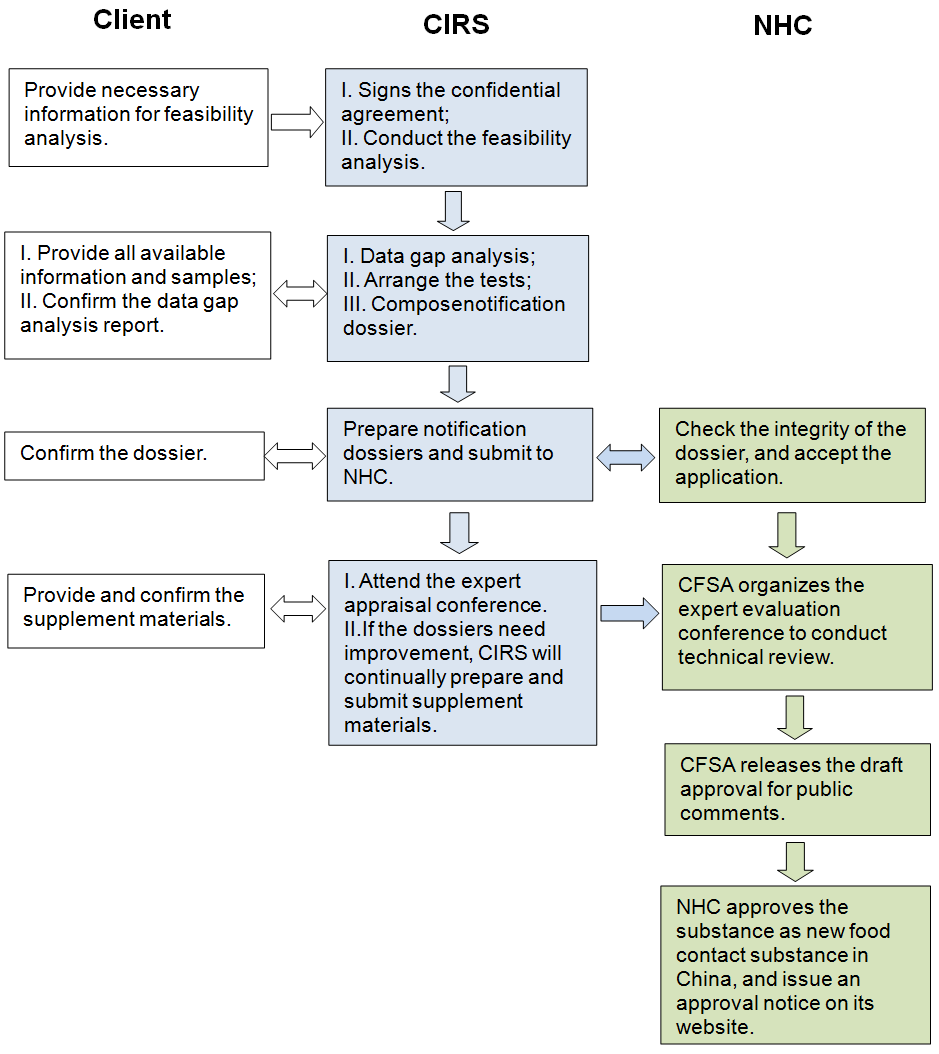
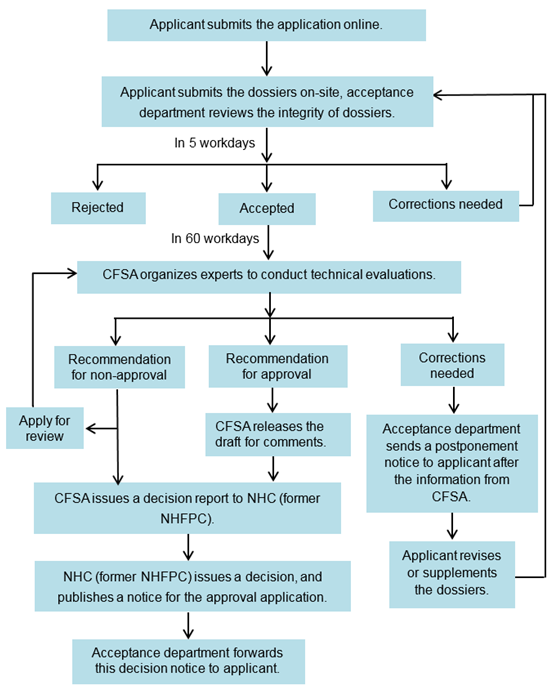
6. Work process of administrative approval
NHC: National Health Commission of the People's Republic of China
NHFPC: The National Health and Family Planning Commission
CFSA: China National Center for Food Safety Risk Assessment
7. Our services
1. New food contact substance notification
- Feasibility analysis
- Notification dossier preparation and submission;
- Foreign documents translation and notarization;
- Tests arrangements and monitoring;
- Project progress tracking;
- Attend the expert appraisal conference and assist enterprise answer the technical questions;
2. Declaration of Compliance (DoC) of FCM
3. Training on new food contact substance notification
4. Other customized service
If you have any other questions, please feel free to contact us at service@cirs-group.com

