1. Background
With the release of Health Food Registration Review Rules (hereinafter referred to as Review Rules) on November 17, 2016, and the Health Food Registration Application Service Guideline on December 23, 2016, the health food registration (Blue hat) under the new regulation has already been started in China. The Review Rules is the normative document when CFDA carry out the registration review, therefore, applicants shall pay attention to this document and comply with the every specific requirement when preparing the application dossier. In order to help applicants better understand the Review Rules, CIRS has made a comprehensive interpretation on it and listed the highlights below.
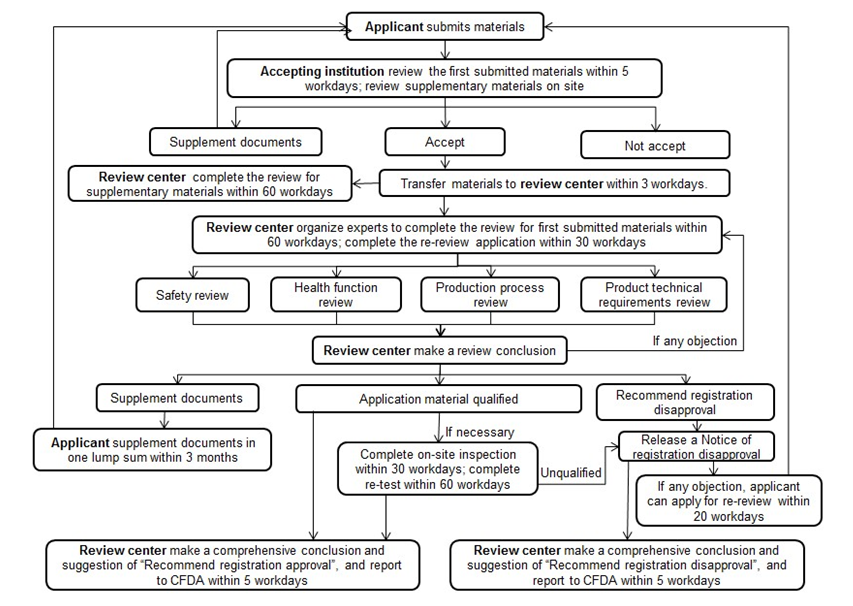
2. Review Procedure of Health Food Registration
3. Key Points in Safety Review
S.N. | Review key points | Main requirements |
1 | Usage basis of raw materials | a) General food, new food raw materials, “homology food and medicine”, “proposed include into health food raw material directory”, etc., all can be used as the legal basis. b) If the raw material is a kind of new health food raw material (without usage basis currently), concerning this new material, the safety evaluation materials, toxicity test report, production process, quality requirements and test report shall be provided, in accordance with new food raw material safety review rules. PS. If the material belongs to part a, but used the production process that may cause significant changes to the substantial basis, it is also classified as new health food raw material. For example, fermented product of Ganoderma lucidum that are produced by enzymolysis, is different from Ganoderma lucidum itself. |
2 | Product formula compatibility and dosage basis | a) The theory basis, reference basis and test data shall support the product safety. b) For example, if the raw material is a new health food raw material, or the dosage is significantly more than the recognized safe dosage, generally, the literatures amounts concerned the dosage safety of the raw material shall be ≥ 5, among them, the scientific literature amounts shall be ≥ 3. |
3 | Product safety evaluation tests | a) Generally, if the product is manufactured by regular production process, the oral acute toxicity test, three terms of hereditary toxicity test, 28 days’oral toxicity test shall be conducted. According to the test results, to determine whether to add the 90 days’ oral toxicity test, teratogenicity test, reproductive toxicity test, chronic toxicity and carcinogenicity test and metabolism test. b) In principle, the test samples shall be integrated packaged. If the no-finalized samples are required for the test, the manufacturing and handling process, as well as the sample preparation requirements and rationality explanation issued by food test agency shall be provided. |
4. Key Points in Health Function Review
S.N. | Review key points | Main requirements |
1 | Function basis for main raw materials, and necessity for other ingredients’ compatibility | a) Generally, for the health function that has be recognized, and there are similar registered health food formulas, the relevant function literatures amount shall be ≥3. b) Generally, if the raw material is a new health food raw material, or the dosage is significantly less than the recognized function dosage, the literatures amounts concerned the dosage function of the raw material shall be ≥ 5, among them, the scientific literature amounts shall be ≥ 3. c) Applicant shall focus on clarifying the functional component and dosage of the raw material, and the does-effect relationship. |
2 | Product formula compatibility and dosage function basis | |
3 | Health function evaluation test and population consumption evaluation materials | a) The health function evaluation animal test shall be conducted at the same test agency with the product safety evaluation test. b) The health function evaluation animal test shall use the same sample with the safety evaluation test, which shall be one batch of those 3 batches samples used for functional component test, hygiene test and stability test. |
5. Key points in Production Process Review
S.N. | Review key points | Main requirements |
1 | Production process research materials | a) For domestic products, the production verification data and self-inspection report at least from 3 batches pilot or above level scales product shall be provided. b) For the first time imported products, if the production research information at lab and pilot scales is integrated, the production verification data and self-inspection report at least from 3 batches from scale-up batches products shall be provided. c) If above information is not integrated for the first time imported products, the production verification report and self- inspection report at least from 10 scale-up batches product issued by oversea manufacturer or trader shall be provided. d) If there is no applicable nation standard, local standard or industry standard for the raw material, the specific preparation technology, process description and production reasonability basis shall all be provided. |
2 | Production process materials | It refers to the production flowchart and description, which includes the main procedures, key technical control points and key technical parameters and the relevant description. All of them shall conform to the production research results. |
6. Key Points in Product Technical Requirement Review
S.N. | Review key points | Main requirements |
1 | Determination basis on the quality requirements of all raw materials and excipients | a) The functional component index shall be the specific component of the main raw material with stable properties and accurate quantity, which shall associate with the health function. b) For multi- raw materials composition product, applicant shall select multiple functional component indexes, in accordance with the active component, specific component, extract technology, composition feature of each raw material.
|
2 | Determination on the test method of each index | All test methods shall be scientific, applicable and repeatable.
a) Generally, test method of physical and chemical index, microbial index shall be national standards, local standards, industry standards or technical guidelines. b) If there is no above method, the detailed test methods and the related method research materials shall be provided.
|
3 | Test reports of functional component, hygiene and stability | Test reports of functional component, hygiene and stability for 3 batches samples shall be provided, and the reports can meet the requirements current technical guidelines and national standards. |
7. Influence and Suggestions
- Under the previous health food registration regulations, applicants can supplement materials for several times, which provide many chances for the product. However, under the new regulations, applicants are required to supplement materials in one lump sum. This change will undoubtedly greatly strengthen the quality requirements on the submitted materials.
- The test reports can be issued by the legally qualified food test agency under the new regulations, and no longer limited to the agencies selected by CFDA. It means that applicants will have more choices when carry out the whole tests. However, for the moment, as the test abilities of the third part food test agencies may have not been enlarged or mature, the important tests are still suggested to conduct under the agencies selected by CFDA.
- When come into the re-test procedure, applicants will have no idea about the re-test agency, which means, it will be impossible to discuss or explain the details of your test methods with them. Therefore, as we mentioned above, the test methods shall be fully applicable and repeatable. Otherwise, the re-test results may be failed just because of the immature methods.
- The relevant content on the label and instruction manuscript will be reviewed by all 4 expert groups from different aspects. For example, the health function expert group will review if the proposed raw materials, excipients, suitable crowds, unsuitable crowds, health function, direction and intake amount etc. are in consist with health function materials or not. Therefore, applicants shall propose the contents based on scientific basis, and pay more attention to the material consistency.
Please click here for more information on health food registration and filing in China.
If you have any needs or questions, please contact us at service@cirs-group.com.

