15 December 2015, CFDA (China Food and Drug Administration) issued “the Notice about the Related Items of Production License of Cosmetics (No 265, 2015)” and “the Notice about How to Better Implement the Relevant Work of Production License of Cosmetics”. The Noticementioned CFDA’s documents that “the Work Specification of Production License of Cosmetics”and“the Key Inspection Points ofProduction License of Cosmetics”, which marked the formal implementation of certificates’ combination. After newly rules coming into force,how to update the old licenses? And how to apply for new Production License of Cosmetics for new manufacturers?
1. Three deadlines and one additional category.
1. Manufacturers are not allowed to produce if they haven’t obtained the new Production License of Cosmetics before 1 January 2017.
2. Since 1 Jan2016, new manufacturers can apply for Production License of Cosmetics in provincial FDA and manufacturers with old licenses can apply for exchanging.
3. Since 1 July, cosmetic products have to indicate new production license information on their packages.
4. The production of oral cavity care products would be regulated by Production License of Cosmetics.
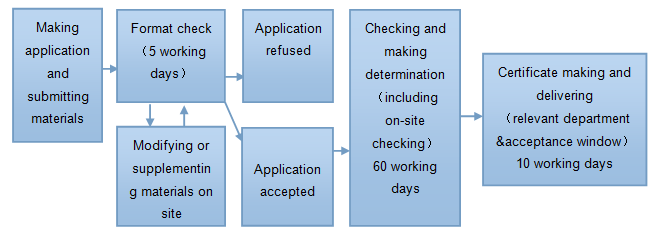
2. The applying procedures of new Production License of Cosmetics
Required materials when applying for Production License of Cosmetics:
No. | The name of materials |
1 | Application form of Production License of Cosmetics |
2 | Ichnography of factory (including the environmental and hygienic situation of 30 meters’ range around the factory), workshop(including functional workshop layout), inspection department and buildings in factory. |
3 | Production equipmentdeployment diagram |
4 | The copy of business license |
5 | Legitimacy verification for the use of production place |
6 | The copy of ID card of legal representative |
7 | For application entrusted, the copy of ID cards of legal representative and agentand a letter of authorization are required. |
8 | Relevant documents of quality control |
9 | Brief description and diagramof the process |
10 | Decoration instructions |
11 | Testing report to verify the manufacturing environment meets the requirement. The report shall be issued within 1 year by state-certifiedtesting institute. |
12 | Manufacturers should make self-inspection according to “the Key Inspection Points of Production License of Cosmetic”, and write inspection report |
13 | Other materials required by provincial FDA. |
3. CIRS comments
1. The National Industrial Production Permit and the Hygiene License for Production Enterprises of Cosmetics combined into the Production License of Cosmetics. This means that the check procedure will be simplified and the period of validate will be prolonged. The new certificate will enjoy 5 years period.
2. “The Key Inspection Points of Production License of Cosmetic” also be regarded as Chinese Good Manufacturing Practice of Cosmetics, that is Chinese GMPC. For manufacturers who haven’t implemented global GMPC or ISO22716, the application for newProduction License of Cosmetics might to be confronted with the difficulty.
3. Software requirements: the new certificate puts forward strict requirements on quality management system of manufacturers. This will be carried out during the whole process of raw material purchase, manufacture, inspection, storage and sales. Manufacturers shall establish traceability management system for product quality control, and monitoring system for adversereactioneffect.
4. Hardware requirements: new certificate has more detailed requirements on workshop and production equipment. The workshop shall be classified as clear area, should-be-clear area and general area, according to product process environment control requirements. The filling room and cleaning container storageroom used for the production of eye-area skincare products and infant or children’s skincare products should meet level 300,000clean requirements.

