On 27 July, 2017, Guangdong
FDA issued the first domestic health food filing product of “SUNOTA Folic Acid
Tablets”. Guangzhou SUNOTA Health Technology Co., Ltd got the first health
food filing certificate in China. Thereafter, different provincial FDAs successively
released the Filing Notices of health food products, and the number of filing
products is increasing rapidly. At present, at least hundreds of domestic
nutrient supplements have got the filing approval. However, since the ways and
frequencies to publish filing products, and the published information are both
different in different provincial FDAs, the complete information of the
approved domestic filing products are still unavailable.
In order to help relevant
enterprises have an overview of filing products in China, CIRS checked the
websites of every provincial FDA in China, and counted the data of the filing
products that have been officially released to the public for your reference.
1.
Approval numbers of domestic health food filing products
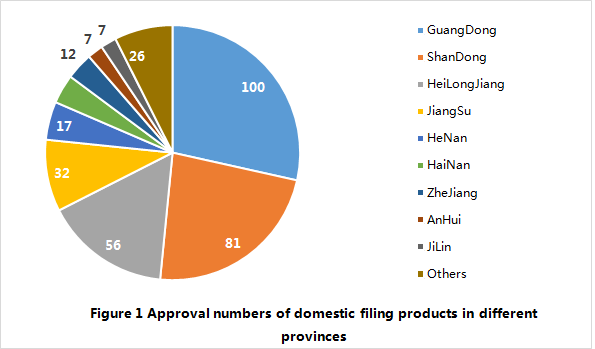
Up to 28 February 2018, a total of 351 domestic filing products have been officially released to the public by different provincial FDAs in China. Among them, 100 products are from Guangdong province, which accounts for 28% of total. In addition, there are more than 50 products released to the public in Shandong province and Heilongjiang province as well.
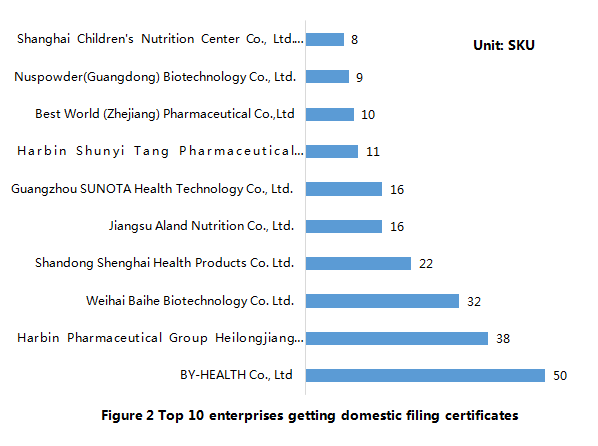
The top 10 enterprises that get the domestic health food filing certificates are as follows. Among them, By-HEALTH Co., Ltd ranks No. 1, with 50 filing nutrient supplements (27 products of them are supplying multiple vitamins-minerals).
2.
Specific information of the approved filing products
2.1 Numbers of approved filing products under different health functions
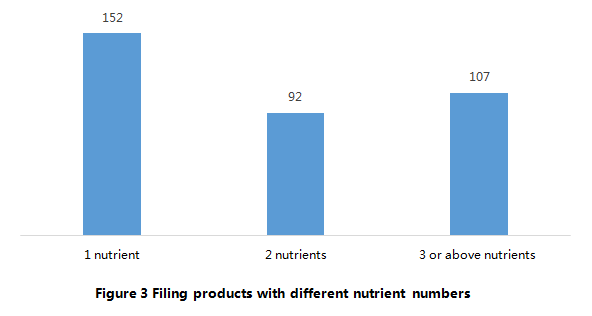
According to Figure 3, among the 351 approved filing products, 152 products are intended to “supply 1 nutrient”, which account for 43% of total. In addition, the products that intended to “supply 1 nutrient” and “supply 2 nutrients” account for 70% of total products. In summary, the health functions of the most approved products are simple.
In terms of the 152 filing
products that only supply 1 nutrient, the health functions ranked the forefront
are as follows. Among them, the health function of “supply vitamin C” takes the
first place, which account for 30% of the 152 filing products.
Health function | Product numbers (SKU) |
Supply vitamin C | 46 |
Supply calcium | 21 |
Supply zinc | 18 |
Supply vitamin D | 16 |
Supply selenium | 11 |
Supply vitamin E | 11 |
In terms of the 92 filing products that supply 2 nutrients, 33 products of them are intended to “supply vitamin D + calcium”, which account for 36% of them. This shows that with the increasing number of health food filing products, enterprises will also face the problem of product homogenization.
2.2 Dosage forms of the approved filing products
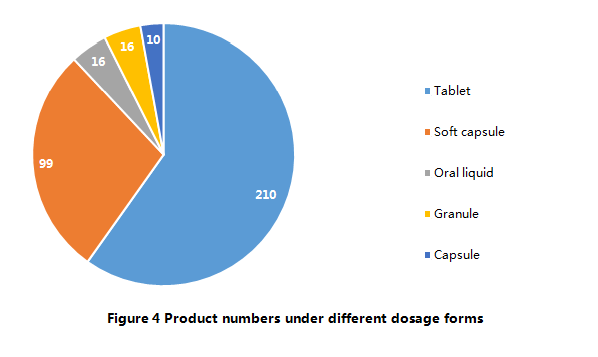
Currently, according to Main Production Processes of Health Food
Filing Products (Trial), there are only 5 dosage forms allowed to be used
for health food filing, namely, tablets, hard capsules, soft capsules, oral
liquids, and granules. The 351 approved filing products have covered all 5
dosage forms. Among them, 210 products are tablets (including tablets, chewing
tablets, effervescent tablets), which account for 60% of total. On the other
hand, products with the dosage form of hard capsules are
in the least. At present, there are only 10 hard capsule filing products
available.
3. Summary and suggestions
Health food filing is
unprecedented in China. It is developed with the goal of simplifying the
procedures of administrative examination, which will help to invigorate the market,
and reduce the enterprise burden. With the approval of first domestic filing
product, hundreds of products have got the filing approval in less than one
year. It can be expected that with the expanding of Health Food Raw Material Directory, more products will turn to
health food filing. However, from another point of view, the filing system may
lead manufacturers to producing similar products because of the restrictive raw
materials and excipients, and the competition will get tougher.
Therefore, in order to enter the China market in the earliest, enterprises who
intend to apply for health food filing are suggested to carry out the
preparation as soon as possible.
For the situation of imported health food filing products, please kindly click “18 Imported Nutrition Supplements have Obtained the Health Food Filing Certificates in China” for detailed information. Up to 4 March, there is no additional product released to the public.
If you have any needs or questions, please contact us at service@cirs-group.com.

