Since Guangdong FDA issued the first domestic health food filing product on 27 July, 2017, more than one thousand products have obtained the health food filing approval by the end of 2018. In order to help relevant enterprises have an overview of this kind of products in China, CIRS counted the data of the approved domestic and imported health food filing products from 27 July, 2017 to the end of 2018, and made an analysis for your reference.
1. The Number of Approved Health Food Filing Products per Month
Up to the end of 2018, the number of approved domestic health food filing products has reached 1750, and the number of approved imported health food filing products has reached 33.
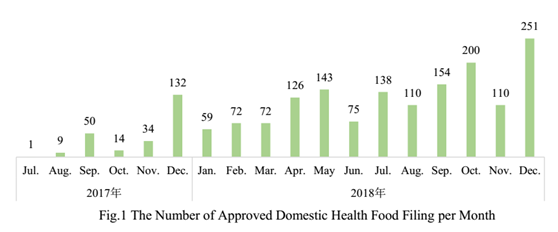
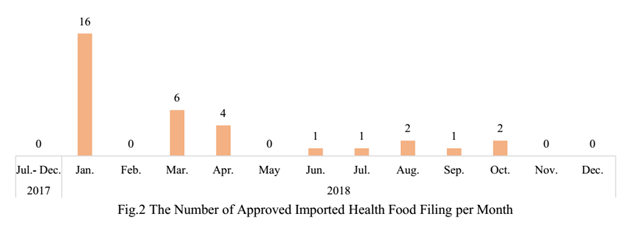
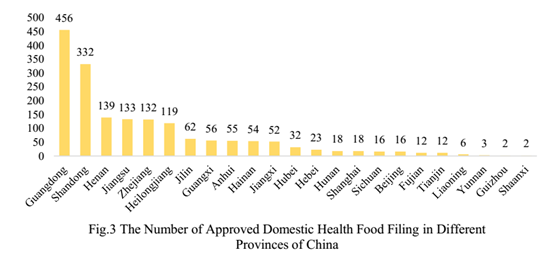
2. The Number of Approved Health Food Filing Products in Different Regions
The number of approved domestic health food filing products is varied in different provinces in China. Guangdong and Shandong rank the first and second place respectively with 456 and 332 approved health food filing products each, which account for 26.1% and 19.0% of total respectively.
The oversea applicants who come from the United States, Hong Kong, Australia, South Korea, New Zealand and Canada have obtained health food filing certificates, and 18 of total approved imported nutrition supplements are from American companies.
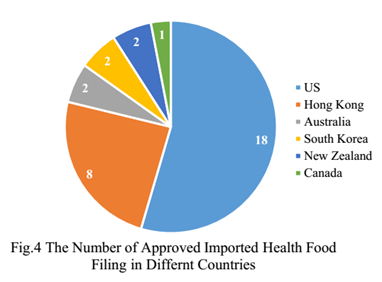
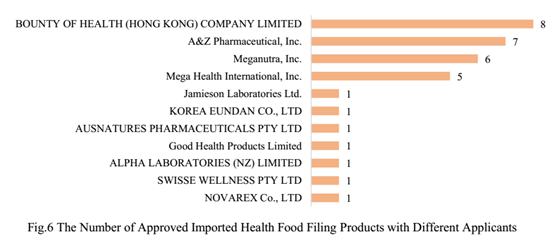
3. The Number of Approved Health Food Filing Products with Different Applicants
There are 248 domestic health food manufacturers who have obtained health food filing certificates. Among them, Weihai Baihe Biology Technology Co., Ltd., has got the largest number of filing certificates of health foods (80), followed by By-Health Co., Ltd. and HPGC Heilongjiang Tongtai Parmaceutical Co., Ltd. with the number of 72 and 70, respectively.

There are 11 oversea companies who have got health food filing certificates, BOUNTY OF HEALTH (HONG KONG) COMPANY LIMITED, has got the largest number of health food filing certificates (8), followed by A & Z Pharmaceutical, Inc. and Meganutra, Inc., which are 7 and 6 respectively.
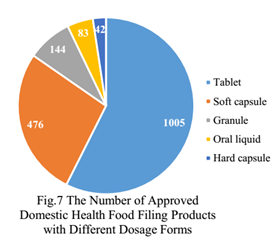
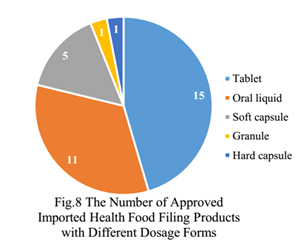
4. The Number of Approved Health Food Filing Products with Different Dosage Forms
There are 5 dosage forms for nutrition supplement which can apply for filing certificate in China currently, namely, tablet, hard capsule, soft capsule, oral liquid and granule. The dosage forms of approved domestic health food filing products are mainly tablets, with the number of 1005, which account for 57.4% of total, followed by soft capsules with the number of 476, which account for 27.2% of total.
The dosage forms of imported health food filing products are mostly tablets, with the number of 15, which account for 45.5% of total, followed by oral liquids (including drops) with the number of 11, which account for 33.3% of total.
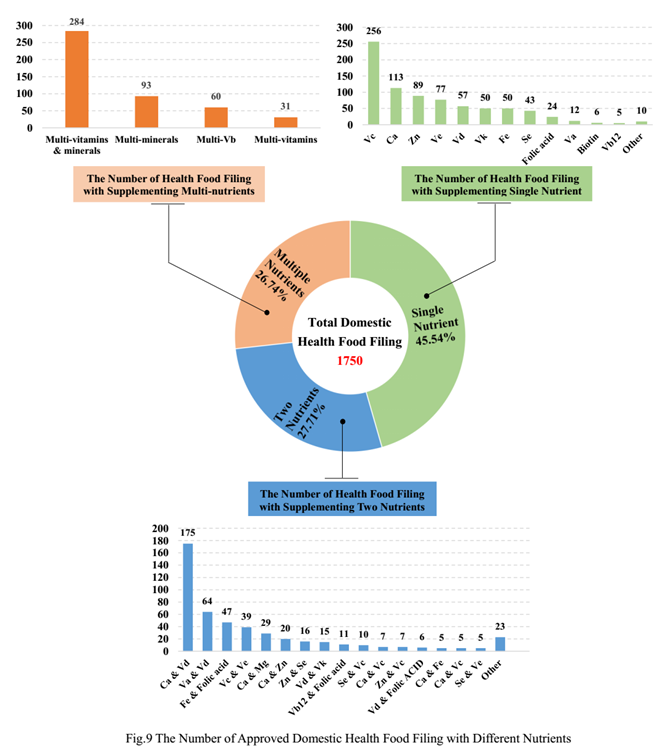
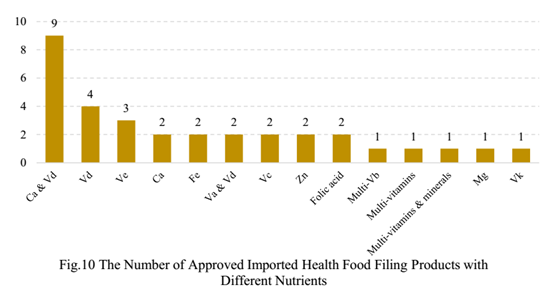
5. The Number of Approved Health Food Filing Products with Different Nutrients
Among domestic health food filing products, the most popular nutrition supplements are Vitamin C supplement, Calcium and Vitamin D supplement, and multi-vitamins and minerals supplement. Among imported health food filing products, the most popular nutrition supplements are Calcium and Vitamin D supplement, and Vitamin D supplement.
Health food filing is unprecedented in China, it is developed with the goal of simplifying the procedures of administrative examination, which will help to invigorate the market, and reduce the enterprises’ burden. From 27 July, 2017 to the end of 2018, more than one thousand nutrition supplements have obtained filing certificates. However, from another point of view, the filing system may lead manufacturers to producing similar products due to the restrictive raw materials and excipients, and the competition will be getting tougher. Therefore, how to highlight the advantages of products is where enterprises need to consider. It can be expected that with the expanding of Health Food Raw Material Directory, Excipient Directory and filing dosage forms, more products will turn to health food filing, and enterprises will have more choices in terms of product formula and dosage forms.
If you have any needs or questions, please contact us at service@cirs-group.com.

