In order to help relevant enterprises have an overview of this kind of products in China, CIRS counted the data of the approved domestic and imported health food filing products from 2017 to July 30th, 2020, and made an analysis for your reference.
1. The Number of Approved Filed Health Foods in China
Since former Guangdong FDA issued the first domestic
filed health food on 27th July, 2017, the number of approved filed
health foods has reached 4087 by July 30th, 2020. The quantity of
domestic and imported filed health foods is 3983 and 104 respectively.
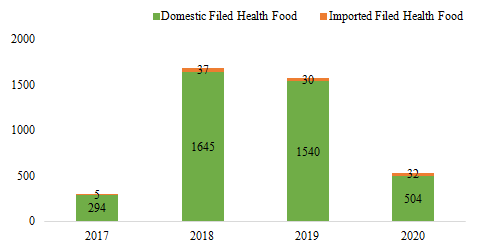
Fig.1 The Number of Approved Filed Health Food from 2017 to 2020
2. The Number of Approved Filed Health Foods in Different Regions
2.1 Domestic Filed Health Food
The number of approved domestic filed health foods is varied in different provinces in China. Shandong and Guangdong rank the first and second place respectively with the number of 773 and 606, which account for 19.41% and 15.21% of total domestic products, respectively.
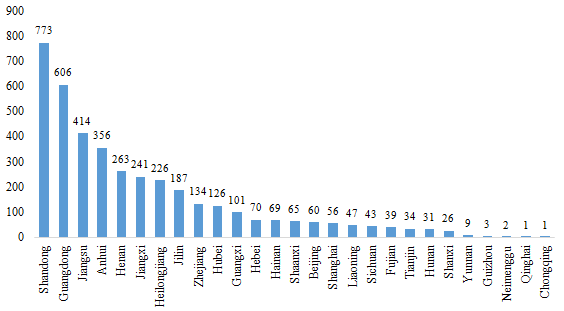
Fig.2 The Number of Domestic Filed Health Food in Different Regions
2.2 Imported Filed Health Food
The applicants who come from the United States, Canada, Australia, Hong Kong-China, New Zealand, South Korea, Germany, Finland, Israel and the United Kingdom have obtained filing certificates, and 60 of total approved imported nutrition supplements are from American companies. It is the first time for Finland, UK and Israeli companies to get filing certificates.
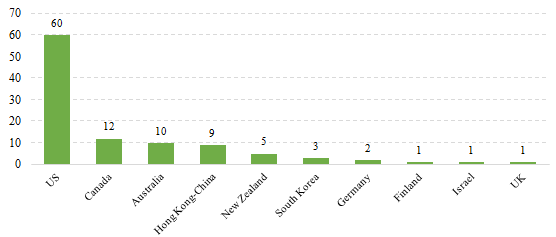
Fig.3 The Number of Imported Filed Health Food in Different Countries/Regions
3. Enterprises Obtaining Health Food Filing Certificates
3.1 Domestic Filed Health Food
There are 432 domestic health food manufacturers who have obtained health food filing certificates. Among them, Weihai Baihe Biology Technology Co., Ltd. has got the largest number of filing certificates (221), followed by Weihai Nanbowan Biotechnology Co., Ltd. and By-Health Co., Ltd. with the number reaching 110 and 89, respectively.
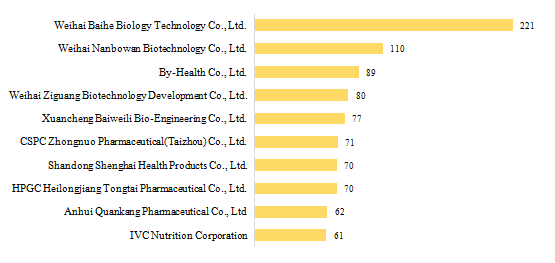
Fig.4 Top 10 Enterprises in Obtaining Domestic Health Food Filing Certificates
3.2 Imported Filed Health Food
There are 27 oversea companies who have got health food filing certificates, Mega Health International, Inc. has got the largest number of filing certificates (17). Jamieson Laboratories Ltd. ranks the second place with the number reaching 12, followed by A&Z Pharmaceutical, Inc., which numbered 10.
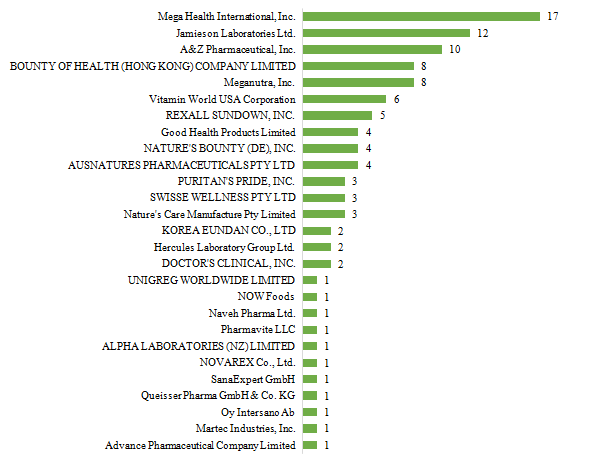
Fig.5 The Number of Approved Imported Health Food Filing Products with Different
4. The Number of Approved Filed Health Food with Different Dosage Forms
There are 5 dosage forms for nutrition supplement which can apply for filing certificate in China currently, namely, tablet, hard capsule, soft capsule, oral liquid (including drops) and granule.
4.1 Domestic Filed Health Food
The dosage forms of approved domestic filed health food are mainly tablets with the number reaching 2283, accounting for 57.32% of total domestic products. Among them, the number of products with the dosage of swallowing tablets and chewable tablets is the largest, which has reached 978 and 959. However, the quantity of products with effervescent tablets and buccal tablets is only 206 and 140 respectively.
Capsule includes soft capsule and hard capsule. The number of the products with capsules is 1145. The quantity of soft capsule products is far higher than that of hard capsule products, which is 1051 and 94 respectively.
In addition, the quantity of oral liquid (including drops) and granule products is 262 and 293. There are only 50 dorps products.
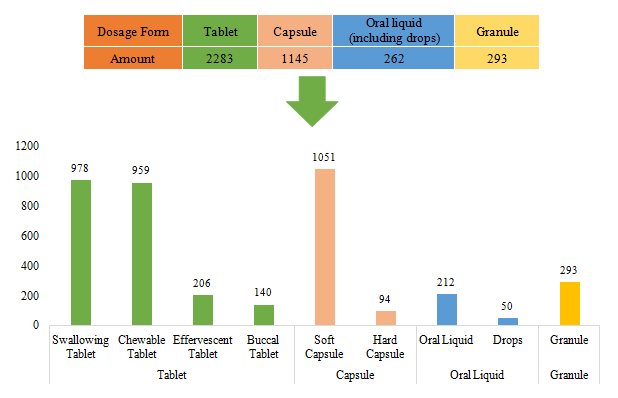
Fig.6 The Number of Approved Domestic Filed Health Food with Different Dosage Forms
4.2 Imported Filed Health Food
The dosage forms of approved imported filed health food are mostly tablets with the number reaching 60, accounting for 57.69% of total imported products. Among them, the number of swallowing tablet products and chewable tablet products is largest, and there is only 1 product with the dosage of effervescent tablet. Besides, there is no buccal tablet product currently.
The quantity of capsule products and oral liquid (including drops) products is 26 and 17 respectively. Same as domestic nutrition supplements, the quantity of soft capsule products is far higher than that of hard capsule products. Different from domestic nutrition supplements, the number of imported drops products is more than oral liquid products. There is only 1 granule products for now.
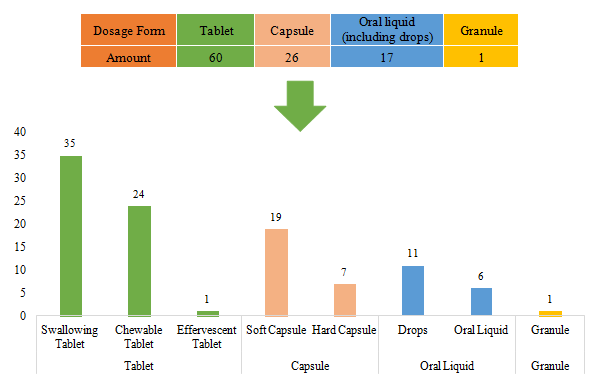
Fig.7 The Number of Approved Imported Filed Health Food with Different Dosage Forms
5. The Number of Approved Filed Health Food with Different Nutrients
5.1 Domestic Filed Health Food
The number of domestic products with supplementing single nutrient is the largest, which is 1791, followed by products with supplementing multiple nutrients and two nutrients.
Among domestic health food filing products, the most popular nutrition supplements are multi-vitamins and minerals supplements, Vitamin C supplements, Calcium and Vitamin D supplements, which is 658, 616 and 379 respectively.
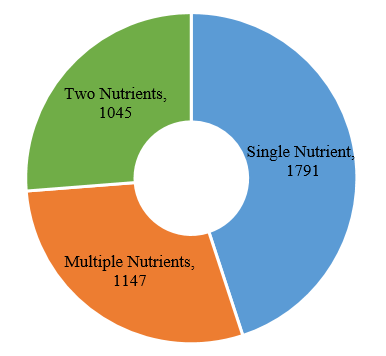
Fig.8 The Number of Approved Domestic Filed Health Food with Different Nutrients
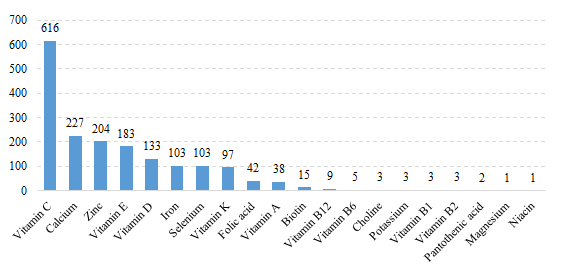
Fig.9 The Number of Domestic Filed Health Food with Supplementing Single Nutrient
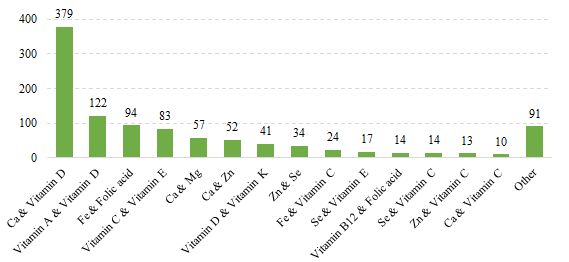
Fig.10 The Number of Domestic Filed Health Food with Supplementing Two Nutrients
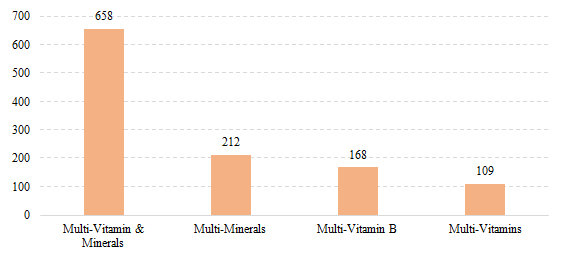
Fig.11 The Number of Domestic Filed
Health Food with Supplementing Multi-Nutrient
PS. Nutrients with less than 10 products are classified as “Other"
5.2 Imported Filed Health Food
Among imported health food filing products, the most popular nutrition supplements are multi-vitamins and minerals supplements, Calcium and Vitamin D supplements, and Vitamin D supplements, which is 27, 12 and 10 respectively.
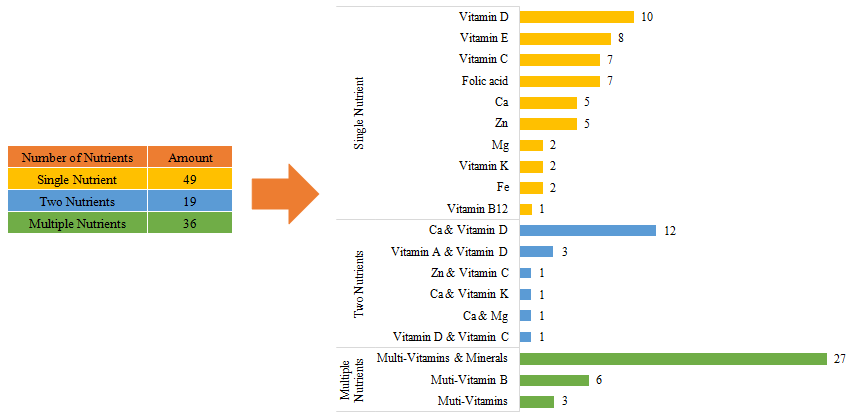
Fig.12 The Number of Approved Imported Filed Health Food with Different Nutrients
Note:
I. The data in this article is from the Special Food Information Query Platform, Center for Food Evaluation of SAMR, and Local Administration for Market Regulation.
II. There may be some omissions in the data of domestic filed health food due to the replacement of new and old websites of government departments after the reform of state institutions, thus the data in this article is for reference only, and please refer to the information published by the government.
III. The Special Food Information Query Platform and Center for Food Evaluation of SAMR lag behind in information release. According to CIRS’s knowledge, the information of some products that have obtained health food filing certificates have not been published on the official website of the government departments. Therefore, the actual record amount of health food exceeds the data listed in this article.
If you have any needs or questions, please contact us at service@cirs-group.com.
