Background:
Chinese government received strong complaints from the Public because of the food safety issues in the past few years. To cope with this, Chinese President Xi Jinping announced the revised Food Safety Law of the People's Republic of China on April 24, 2015. With the implementation of the Food Safety Law, lots of regulations regarding Food, Health food, Infant food, Food for Special Medical Purpose (FSMP) and Food contact material and article (FCM) (click here to find the 52 new GBs released in November, 2016) were released in 2015 and 2016.
Among the variety of FCM regulations and National food safety standards (GB), the most important two for FCM are GB 9685-2016 Standard for the Uses of Additives in Food Contact Materials and Articles and GB 4806.1-2016 General Safety Requirements of Food Contact Materials and Articles which were published on November 18, 2016 and will come into force on October 19, 2017.
GB9685-2016
GB 9685-2016 takes the place of GB 9685-2008, and includes 1294 approved food contact additives. It specifies the principles for uses of additives in FCM, approved additive categories, application scope, and maximum permitted level, specific migration limit or maximum residue limit, total specific migration limit and other restrictions of the additives in FCM. Compared to the 2008 version, the additives and their usage requirements in the new version are listed separately in Table A.1 to Table A.7 of Annex A according to the category of the applicable scope. Take one additive - Butylated hydroxyanisole (CAS No. 25013-16-5) for example as following:
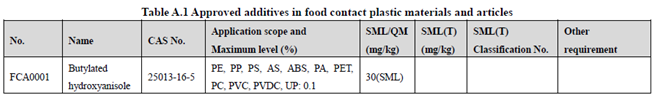
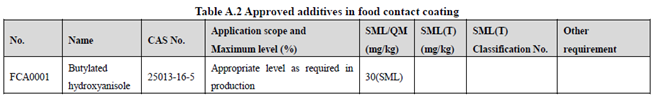
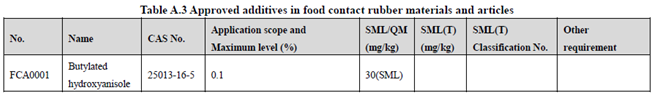
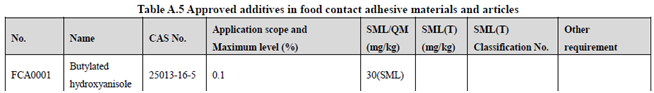
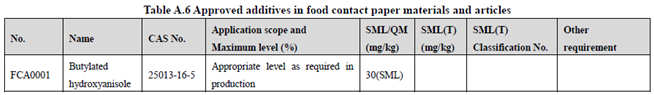
According to the information in the above Tables, Butylated hydroxyanisole (CAS No. 25013-16-5) is permitted in food contact plastic, coating, rubber, adhesive, and paper, but is not allowed in food contact ink or others. Meanwhile, it could be noted that the maximum level of Butylated hydroxyanisole in different FCM is different.
GB4806.1-2016
With growing calls for creating a completed regulatory system for food contact materials and articles in China, only the GB 9685 is insufficient. Therefore, National Health and Family Planning Commission (NHFPC) set up a brand new GB called “GB 4806.1-2016 General Safety Requirements of Food Contact Materials and Articles” which is also a general GB applying to all kinds of FCM. It specifies the basic requirements, restrictions, compliance principle, test methods, traceability and product information for FCM. The most fresh and important points in GB 4806.1-2016 are as follows:
Basic Requirements
- For substance which is not listed in corresponding GBs, but will indirectly contact food with an effective barrier, manufacturers shall evaluate and control the risk, and ensure that the migration of unapproved substance shall not exceed 0.01 mg/kg (Not applicable to carcinogenic, teratogenic, mutagenic substances and nanometer substance).
- The production of food contact materials and their products shall meet the requirements of GB 31603-2015 General Hygienic Practice for Production of Food Contact Materials and Articles.
Restrictions
- The overall migration level, substance usage level, specific migration level, total specific migration level, residue level, etc. in food contact materials and articles should be in accordance with the requirements of relevant GBs.
Compliance Principle
- The use of raw materials in FCMs shall be consistent with the corresponding Product GB and related Notices.
- The use of additives in FCMs should comply with GB 9685 and related Notices.
- FCMs shall be consistent with the corresponding Product national food safety standards.
Test Methods
- The migration test methods of FCMs shall comply with the requirements in GB 31604.1 and GB 5009.156.
- The test methods of other tests for FCMs shall be the GB methods (In the absence of GB methods, other reliable methods can be used).
Traceability
- Manufacturers shall establish a traceability system for FCMs from production to distribution.
- The product information including label, instruction, etc., is mandatory (label is a top priority).
- The product information shall include the product name, material, Declaration of Conformity (DoC) according to relevant GBs, name, address and contact of the producer and (or) distributor, date of manufacture and shelf life (if applicable), etc.
- Finished food contact product shall also be labelled with “food contact use”, “food packaging use” or similar terms, or be printed/pasted with spoon & chopsticks logo (See below picture); Except for products (e.g. chopsticks, woks) which have a clear purpose for food contact use.
Declaration of Conformity (DoC)
For the purpose of evaluating the compliance and safety of food contact materials accurately and delivering product information smoothly, GB 4806.1-2016 pointed out that product Declaration of Conformity (DoC) is mandatory in the supply chain in China.
DoC is available for “Chemical substances” (Chemical substances refers to substances listed in GB 9685 including additives, solvents, colorants, etc. ) which will be used for producing FCM, “Intermediate materials” (Intermediate materials refers to materials such as resins listed in Product GB, premix, etc.) which will be used for producing finished FCM, and “Finished FCM” which will contact with food. Therefore, DoC will be delivered according to the following supply chain:
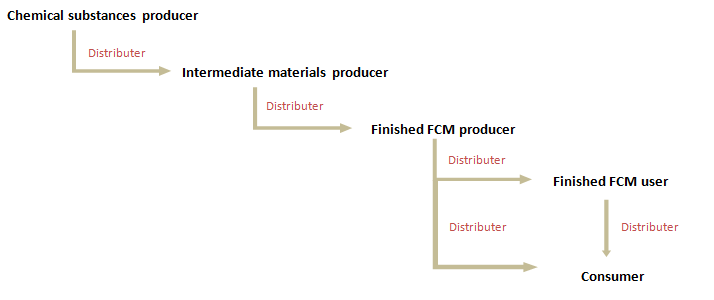
- "Chemical substances” manufacturers shall produce food contact substances according to GB 31603 and prepare the DoC including the application limit and condition of the “Chemical substances” for downstream companies.
- “Intermediate materials” manufacturers shall require the DoCs from “Chemical substances” manufacturers and produce food contact Intermediate materials according to GB 31603 and relevant GBs, then prepare the DoC including the application limit and condition of the “Intermediate materials” for downstream companies.
- “Finished FCM” manufacturers shall require the DoCs from “Chemical substances” and/or “Intermediate materials” manufacturers and produce finished food contact materials and products according to GB 31603 and relevant GBs, then prepare the DoC including the application limit and condition of the “Finished FCM” for downstream users and/or consumers. In addition, the supporting documents of DoC should be kept by the DoC declarant.
Generally, there are two parts in the DoC, one is the basic information of the product, and the other one is the restrictions of the product. Following is a brief example of DoC for product-finished FCM:
Declaration of Compliance (Finished Product)
Product name: | |
Product material: | |
Manufacturer: | |
Contact Information: | |
Declarant: | |
Contact Information: | |
Product Use Condition: | |
…… | |
Regulatory Compliance: | |
Regulatory Compliance Statement: | |
Disclaimer |
Sign or seal:
Issued date:
Annex: Restrictions and compliance statement
1.
2.
......
In addition, before issuing the DoC for finished FCM, the supporting documents shall be prepared. Documents shall include, but shall not be limited to:
- Specifications of materials and additives used in finished FCM
- DoCs of materials and additives from the suppliers
- Product compliance analysis reports
- Test reports
CIRS Services:
1. Product compliance analysis
- Check if all materials and additives are permitted or not in China;
- Check if the level of the materials and additives are permitted or not in China;
- Check if the materials and additives meet the restrictions or not;
2. Testing
- Test in the corresponding product GB (For example, the general test items in GB 4806.11-2016 shall be carried out for Rubber);
- SML/QM test for the restricted materials and additives;
3. Suggest DoC contents/ issue DoC
4. New food contact material and additive registration
If you have any needs or questions, please contact us at service@cirs-group.com.

