From January 25 to January 27, 2018, CFDA Health Food Review Center has published several Notices about the filing information of imported health food. This is the first batch of imported products obtaining the filing certificates, which include 18 nutrition supplements in total. Among them, 5 products got the filing certificates in the end of 2017, and the other 13 products were filed in early 2018.
1. Imported Filing Products Information
According to CIRS count, the 18 approved filing products come from 4 overseas applicants, namely,
- Meganutra,Inc., with 6 products;
- BOUNTY OF HEALTH (HONG KONG) COMPANY LIMITED, with 6 products;
- Mega Health International, Inc., with 5 products;
- A&Z Pharmaceutical, Inc., with 1 product.
Excepting “BOUNTY
OF HEALTH (HONG KONG) COMPANY LIMITED”, the other 3 applicants are all American
enterprises. In addition, the manufacturers of all 18 products are from
America. These show the leading speed of American enterprises in obtaining
imported health food filing approval.
The dosage
forms of the 18 approved products include tablet, soft capsule, drop, oral
liquid, etc. Detailed filing information is as follows:
S.N. | Product name | Applicant | Manufacturer | Filing number |
1 | Nature´s Nutra Zinc | Meganutra,Inc. | Healthy Solutions, LLC | 食健备J201700000001 |
2 | NATURE’S BOUNTY® C | BOUNTY OF HEALTH (HONG KONG) COMPANY LIMITED | Robinson Pharma, Inc. | 食健备J201700000002 |
3 | NATURE’S BOUNTY® Vitamin D Calcium | BOUNTY OF HEALTH (HONG KONG) COMPANY LIMITED | Robinson Pharma, Inc. | 食健备J201700000003 |
4 | NATURE’S BOUNTY®Folic Acid | BOUNTY OF HEALTH (HONG KONG) COMPANY LIMITED | Robinson Pharma, Inc. | 食健备J201700000004 |
5 | NATURE’S BOUNTY®D3 | BOUNTY OF HEALTH (HONG KONG) COMPANY LIMITED | Robinson Pharma, Inc. | 食健备J201700000005 |
6 | D-Cal® Calcium and Vitamin D3 Nutritional Supplement Chewable Tablets | A&Z Pharmaceutical, Inc. | A&Z Pharmaceutical, Inc. | 食健备J201800000001 |
7 | Infantum Zinc | Mega Health International, Inc. | Healthy Solutions, LLC | 食健备J201800000002 |
8 | Infantum Calcium | Mega Health International, Inc. | Healthy Solutions, LLC | 食健备J201800000003 |
9 | Infantum Vitamin D3 | Mega Health International, Inc. | Healthy Solutions, LLC | 食健备J201800000004 |
10 | Nature´s Nutra Vitamin A&D | Meganutra,Inc. | Healthy Solutions, LLC | 食健备J201800000005 |
11 | Infantum Iron | Mega Health International, Inc. | Healthy Solutions, LLC | 食健备J201800000006 |
12 | Nature´s Nutra Sunshine Vitamin D3 | Meganutra,Inc. | Healthy Solutions, LLC | 食健备J201800000007 |
13 | Infantum Vitamin AD | Mega Health International, Inc. | Healthy Solutions, LLC | 食健备J201800000008 |
14 | Nature's Nutra Prenatal Folic Acid | Meganutra,Inc. | Healthy Solutions, LLC | 食健备J201800000009 |
15 | Nature´s Nutra Iron | Meganutra,Inc. | Healthy Solutions, LLC | 食健备J201800000010 |
16 | Nature's Nutra Calcium | Meganutra,Inc. | Healthy Solutions, LLC | 食健备J201800000011 |
17 | NATURE'S BOUNTY®E | BOUNTY OF HEALTH (HONG KONG) COMPANY LIMITED | Robinson Pharma, Inc. | 食健备J201800000012 |
18 | NATURE'S BOUNTY®K2 | BOUNTY OF HEALTH (HONG KONG) COMPANY LIMITED | Robinson Pharma, Inc. | 食健备J201800000013 |
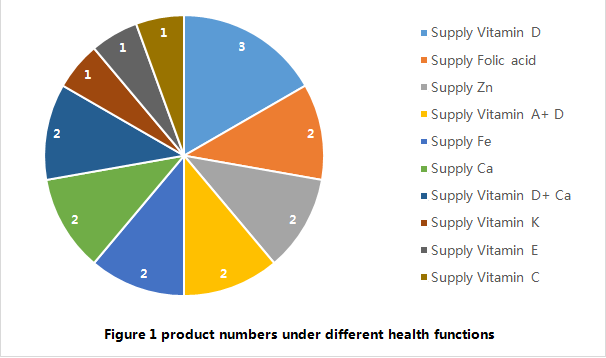
Concerning the health function, 4 approved products are intended to “supply 2 nutrients” (2 products of supplying vitamin D +Ca, 2 products of supplying vitamin A +D), and the other 14 products are all intended to supply single vitamin or mineral. For the moment, there is no imported filing product with the function of “supply multivitamins and/or minerals (3 or more vitamins or minerals) published by CFDA Health Food Review Center. In summary, the health functions of the 18 approved products are simple.
2.
CIRS Comments
Health
food filing is unprecedented in China. It shortens the application time and
reduces the enterprise burden. With the smooth publishing of the first batch of
imported health food filing certificates, CIRS speculates that more and more imported
health foods will get the filing certificates successfully
in the near future.
In order to enter China market as soon as possible with legal status, relevant nutrition supplement enterprises are recommended to further study on the requirements of China health food filing, carry out the filing feasibility analysis before filing application, and submit health food filing application to CFDA at the earliest. More regulatory compliance questions about the health food filing in China, please contact us at service@cirs-group.com.
Reference

